Human autologous mixed lymphocyte reaction as an in vitro model for autoreactivity to apoptotic antigens
- PMID: 12423312
- PMCID: PMC1782802
- DOI: 10.1046/j.1365-2567.2002.01516.x
Human autologous mixed lymphocyte reaction as an in vitro model for autoreactivity to apoptotic antigens
Abstract
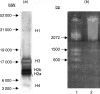
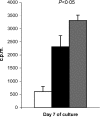
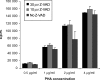
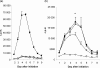
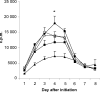
Recent studies have indicated that cells undergoing apoptosis are the source of autoantigens which drive autoimmune responses in systemic lupus erythematosus (SLE). It has been recognized for many years that in vitro stimulation of T cells with irradiated major histocompatibility complex (MHC) class II-bearing autologous cells results in T-cell proliferation with immunological specificity and memory, namely the autologous mixed lymphocyte reaction (AMLR). The nature of the major stimulants in the AMLR is still unclear. We investigated whether apoptotic fragments from irradiated cells act as antigenic stimulators for AMLR or nucleohistone-primed T cells. T-cell proliferation in the primary AMLR was significantly suppressed by the presence of a caspase inhibitor Z-Val-Ala-Asp-CH2F (Z-VAD.fmk), indicating that apoptotic antigens released from irradiated autologous feeder cells act as stimulators of AMLR T cells. This inhibitory effect of Z-VAD was not caused by toxic effects, because the T-cell response to the mitogen phytohaemagglutinin (PHA) was not inhibited by Z-VAD. A nucleohistone preparation was shown to contain antigens that are important in the AMLR, as culture with nucleohistone (but not with thyroglobulin or hen-egg lysozyme) primed T cells to respond with secondary kinetics in a subsequent AMLR that was also suppressed by Z-VAD. Our data provide evidence that the AMLR constitutes a model for the evaluation of cellular and molecular mechanisms that may be relevant to the pathogenesis of SLE and similar autoimmune diseases.
Figures
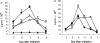

Similar articles
-
Responder cells in the human autologous mixed lymphocyte reaction (AMLR). Characterization and interactions in healthy individuals and patients with systemic lupus erythematosus.Behring Inst Mitt. 1983 May;(72):135-42. Behring Inst Mitt. 1983. PMID: 6242332
-
On the immune reaction to autologous human lymphoblasts: evidence for the stimulation by activating factors rather than induction by autoantigens.Clin Immunol Immunopathol. 1987 May;43(2):229-42. doi: 10.1016/0090-1229(87)90131-0. Clin Immunol Immunopathol. 1987. PMID: 2952382
-
Generation of suppressor cells in the autologous mixed lymphocyte reaction.Clin Exp Immunol. 1981 Oct;46(1):185-95. Clin Exp Immunol. 1981. PMID: 6461452 Free PMC article.
-
Studies of autologous T cell activation in the Kunkel laboratory.Lupus. 2003;12(3):163-9. doi: 10.1191/0961203303lu349xx. Lupus. 2003. PMID: 12708774 Review.
-
The autologous mixed lymphocyte reaction (AMLR) as a possible indicator of immunoregulation in chronic inflammatory periodontal disease.J Clin Periodontol. 1986 Aug;13(7):639-45. doi: 10.1111/j.1600-051x.1986.tb00858.x. J Clin Periodontol. 1986. PMID: 2944916 Review.
Cited by
-
Activation-induced FoxP3 expression regulates cytokine production in conventional T cells stimulated with autologous dendritic cells.Clin Vaccine Immunol. 2012 Oct;19(10):1583-92. doi: 10.1128/CVI.00308-12. Epub 2012 Aug 1. Clin Vaccine Immunol. 2012. PMID: 22855393 Free PMC article.
-
Basophils inhibit proliferation of CD4⁺ T cells in autologous and allogeneic mixed lymphocyte reactions and limit disease activity in a murine model of graft versus host disease.Immunology. 2015 Jun;145(2):202-12. doi: 10.1111/imm.12436. Immunology. 2015. PMID: 25545131 Free PMC article.
-
Efficient replication of human immunodeficiency virus type 1 in resting CD4+ T lymphocytes is induced by coculture with autologous dendritic cells in the absence of foreign antigens.J Virol. 2009 Mar;83(6):2778-82. doi: 10.1128/JVI.01420-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109380 Free PMC article.
-
Directing autoimmunity to nucleoprotein particles: the impact of dendritic cells and interferon alpha in lupus.J Exp Med. 2003 Mar 17;197(6):681-5. doi: 10.1084/jem.20030130. J Exp Med. 2003. PMID: 12642600 Free PMC article. No abstract available.
-
Human immune compartment comparisons: Optimization of proliferative assays for blood and gut T lymphocytes.J Immunol Methods. 2017 Jun;445:77-87. doi: 10.1016/j.jim.2017.03.014. Epub 2017 Mar 21. J Immunol Methods. 2017. PMID: 28336395 Free PMC article.
References
-
- Amoura Z, Piette JC, Chabre H, Cacoub P, Papo T, Wechsler B, Bach JF, Koutouzov S. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus: correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis Rheum. 1997;40:2217–25. - PubMed
-
- Levine JS, Koh JS. The role of apoptosis in autoimmunity: immunogen, antigen, and accelerant. Semin Nephrol. 1999;19:34–7. - PubMed
-
- Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

