Functional analysis of the Bacillus subtilis Zur regulon
- PMID: 12426338
- PMCID: PMC135443
- DOI: 10.1128/JB.184.23.6508-6514.2002
Functional analysis of the Bacillus subtilis Zur regulon
Abstract
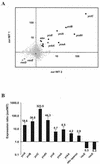
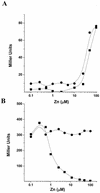
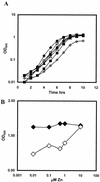
The Bacillus subtilis zinc uptake repressor (Zur) regulates genes involved in zinc uptake. We have used DNA microarrays to identify genes that are derepressed in a zur mutant. In addition to members of the two previously identified Zur-regulated operons (yciC and ycdHI-yceA), we identified two other genes, yciA and yciB, as targets of Zur regulation. Electrophoretic mobility shift experiments demonstrated that all three operons are direct targets of Zur regulation. Zur binds to an approximately 28-bp operator upstream of the yciA gene, as judged by DNase I footprinting, and similar operator sites are found preceding each of the previously described target operons, yciC and ycdHI-yceA. Analysis of a yciA-lacZ fusion indicates that this operon is induced under zinc starvation conditions and derepressed in the zur mutant. Phenotypic analyses suggest that the YciA, YciB, and YciC proteins may function as part of the same Zn(II) transport pathway. Mutation of yciA or yciC, singly or in combination, had little effect on growth of the wild-type strain but significantly impaired the growth of the ycdH mutant under conditions of zinc limitation. Since the YciA, YciB, and YciC proteins are not obviously related to any known transporter family, they may define a new class of metal ion uptake system. Mutant strains lacking all three identified zinc uptake systems (yciABC, ycdHI-yceA, and zosA) are dependent on micromolar levels of added zinc for optimal growth.
Figures




References
-
- Baichoo, N., T. Wang, Y. Rick, and J. D. Helmann. 2002. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol. Microbiol. 45:1613-1629. - PubMed
-
- Beard, S. J., M. N. Hughes, and R. K. Poole. 1995. Inhibition of the cytochrome bd-terminated NADH oxidase system in Escherichia coli K-12 by divalent metal cations. FEMS Microbiol. Lett. 131:205-210. - PubMed
-
- Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

