Low-temperature-induced changes in composition and fluidity of lipopolysaccharides in the antarctic psychrotrophic bacterium Pseudomonas syringae
- PMID: 12426366
- PMCID: PMC135421
- DOI: 10.1128/JB.184.23.6746-6749.2002
Low-temperature-induced changes in composition and fluidity of lipopolysaccharides in the antarctic psychrotrophic bacterium Pseudomonas syringae
Abstract
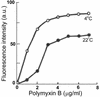
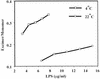
The Antarctic psychrotrophic bacterium Pseudomonas syringae was more sensitive to polymyxin B at a lower (4 degrees C) temperature of growth than at a higher (22 degrees C) temperature. The amount of hydroxy fatty acids in the lipopolysaccharides (LPS) also increased at the lower temperature. These changes correlated with the increase in fluidity of the hydrophobic phase of lipopolysaccharide aggregates in vitro.
Figures

References
-
- Brade, H., and C. Galanos. 1982. Isolation, purification, and chemical analysis of the lipopolysaccharide and lipid A of Acinetobacter calcoaceticus NCTC 10350. Eur. J. Biochem. 122:233-237. - PubMed
-
- Brandenburg, K., and U. Seydel. 1984. Physical aspects of structure and function of membranes made from lipopolysaccharides and free lipid A. Biochim. Biophys. Acta 775:225-238.
-
- Brandenburg, K., and U. Seydel. 1990. Investigation into the fluidity of lipopolysaccharide and free lipid A membrane systems by Fourier-transform infrared spectroscopy and differential scanning calorimetry. Eur. J. Biochem. 191:229-236. - PubMed
-
- Carty, S. M., K. R. Sreekumar, and C. R. H. Raetz. 1999. Effect of cold shock on lipid A biosynthesis in Escherichia coli. Induction at 12oC of an acyltransferase specific for palmitoleoyl-acyl carrier protein. J. Biol. Chem. 274:9677-9685. - PubMed
-
- Emmerling, G., U. Henning, and T. Gulik-Krzywicki. 1977. Order-disorder conformational transition of the hydrocarbon chains in lipopolysaccharide from Escherichia coli. Eur. J. Biochem. 78:503-509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

