Tissue factor deficiency causes cardiac fibrosis and left ventricular dysfunction
- PMID: 12426405
- PMCID: PMC137717
- DOI: 10.1073/pnas.242501899
Tissue factor deficiency causes cardiac fibrosis and left ventricular dysfunction
Abstract
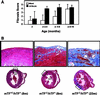
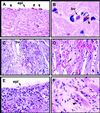
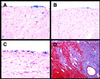
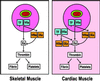
Exposure of blood to tissue factor (TF) activates the extrinsic (TF:FVIIa) and intrinsic (FVIIIa:FIXa) pathways of coagulation. In this study, we found that mice expressing low levels of human TF ( approximately 1% of wild-type levels) in an mTF(-/-) background had significantly shorter lifespans than wild-type mice, in part, because of spontaneous fatal hemorrhages. All low-TF mice exhibited a selective heart defect that consisted of hemosiderin deposition and fibrosis. Direct intracardiac measurement demonstrated a 30% reduction (P < 0.001) in left ventricular function in 8-month-old low-TF mice compared with age-matched wild-type mice. Mice expressing low levels of murine FVII ( approximately 1% of wild-type levels) exhibited a similar pattern of hemosiderin deposition and fibrosis in their hearts. In contrast, FIX(-/-) mice, a model of hemophilia B, had normal hearts. Cardiac fibrosis in low-TF and low-FVII mice appears to be caused by hemorrhage from cardiac vessels due to impaired hemostasis. We propose that TF expression by cardiac myocytes provides a secondary hemostatic barrier to protect the heart from hemorrhage.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

