Nitric oxide inhibits irreversibly P815 cell proliferation: involvement of potassium channels
- PMID: 12427251
- PMCID: PMC6495850
- DOI: 10.1046/j.1365-2184.2002.00240.x
Nitric oxide inhibits irreversibly P815 cell proliferation: involvement of potassium channels
Abstract
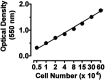
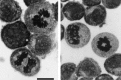
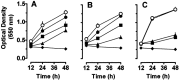
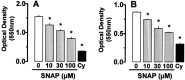
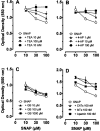
Nitric oxide (NO) has been shown to inhibit both normal and cancer cell proliferation. Potassium channels are involved in cell proliferation and, as NO activates these channels, we investigated the effect of NO on the proliferation of murine mastocytoma cell lines and the putative involvement of potassium channels. NO (in the form of NO donors) caused dose-dependent inhibition of cell proliferation in the P815 cell line inducing growth arrest in the mitosis phase. Incubation with NO donor for 4 or 24 h had a similar inhibitory effect on cell proliferation, indicating that this effect is irreversible. The inhibitory effect of NO was completely prevented by the blockade of voltage- and calcium-dependent potassium channels, but not by blockade of ATP-dependent channels. NO inhibition of cell proliferation was unaffected by guanylate cyclase and by cytoskeleton disruptors. Therefore, NO inhibits cell proliferation irreversibly via a potassium channel-dependent but guanylate cyclase-independent pathway in murine mastocytoma cells.
Figures

Similar articles
-
Membrane potassium currents in human radial artery and their regulation by nitric oxide donor.Cardiovasc Res. 2006 Jul 15;71(2):383-92. doi: 10.1016/j.cardiores.2006.04.002. Epub 2006 Apr 21. Cardiovasc Res. 2006. PMID: 16716281
-
Involvement of soluble guanylate cyclase and calcium-activated potassium channels in the long-lasting hyporesponsiveness to phenylephrine induced by nitric oxide in rat aorta.Naunyn Schmiedebergs Arch Pharmacol. 2000 May;361(5):477-83. doi: 10.1007/s002100000216. Naunyn Schmiedebergs Arch Pharmacol. 2000. PMID: 10832600
-
Participation of the nitric oxide-cyclic GMP-ATP-sensitive K(+) channel pathway in the antinociceptive action of ketorolac.Eur J Pharmacol. 2001 Aug 24;426(1-2):39-44. doi: 10.1016/s0014-2999(01)01206-7. Eur J Pharmacol. 2001. PMID: 11525769
-
Major potassium conductance in type I hair cells from rat semicircular canals: characterization and modulation by nitric oxide.J Neurophysiol. 2000 Jul;84(1):139-51. doi: 10.1152/jn.2000.84.1.139. J Neurophysiol. 2000. PMID: 10899192
-
NO-induced relaxation of labouring and non-labouring human myometrium is not mediated by cyclic GMP.Br J Pharmacol. 2001 Sep;134(1):206-14. doi: 10.1038/sj.bjp.0704226. Br J Pharmacol. 2001. PMID: 11522613 Free PMC article.
References
-
- Albina JE, Reichner JS (1998) Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis. Cancer Metastasis Rev. 17, 39. - PubMed
-
- Arnelle DR, Stamler JS (1995) NO+, NO· and NO− donation by S‐nitrosothiols: implications for regulation of physiological functions by S‐nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 318, 279. - PubMed
-
- Beckman JS, Koppenol WH (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am. J. Physiol. 271, C1424. - PubMed
-
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA (1994) Nitric oxide directly activates calcium‐dependent potassium channels in vascular smooth muscle. Nature 362, 850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

