Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes
- PMID: 12427823
- PMCID: PMC6757830
- DOI: 10.1523/JNEUROSCI.22-22-09679.2002
Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes
Abstract
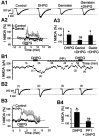
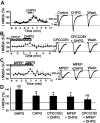
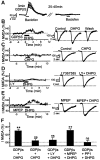
Molecular processes regulating the gain of NMDA receptors modulate diverse physiological and pathological responses in the CNS. Group I metabotropic glutamate receptors (mGluRs), which neighbor NMDA receptors and which can be coactivated by synaptically released glutamate, couple to several different second messenger pathways, each of which could target NMDA receptors. In CA3 pyramidal cells we show that the activation of mGluR1 potentiates NMDA current via a G-protein-independent mechanism involving Src kinase activation. In contrast, mGluR5-mediated enhancement of NMDA current requires G-protein activation, triggering a signaling cascade including protein kinase C and Src. These results indicate that one neurotransmitter, glutamate, can activate two distinct and independent signaling systems to target the same effector. These two pathways are likely to contribute significantly to the highly differentiated control of NMDA receptor function.
Figures


References
-
- Alagarsamy S, Sorensen SD, Conn PJ. Coordinate regulation of metabotropic glutamate receptors. Curr Opin Neurobiol. 2001;11:357–362. - PubMed
-
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signaling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–342. - PubMed
-
- Aniksztejn L, Bregestovski P, Ben-Ari Y. Selective activation of quisqualate metabotropic receptor potentiates NMDA but not AMPA responses. Eur J Pharmacol. 1991;205:327–328. - PubMed
-
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
