Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling
- PMID: 12427829
- PMCID: PMC6757831
- DOI: 10.1523/JNEUROSCI.22-22-09742.2002
Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling
Abstract
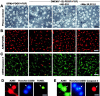
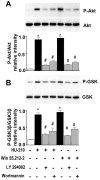
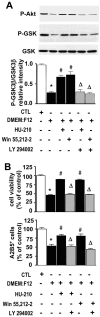
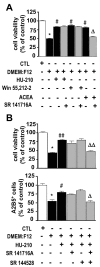
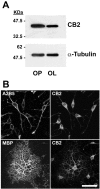
Cannabinoids exert pleiotropic actions in the CNS, including the inhibition of inflammatory responses and the enhancement of neuronal survival after injury. Although cannabinoid receptors are distributed widely in brain, their presence has not been investigated previously in oligodendrocytes. This study examined the expression of cannabinoid type 1 (CB1) receptors in rat oligodendrocytes in vivo and in culture and explored their biological function. Expression of CB1 receptors by oligodendrocytes was demonstrated immunocytochemically in postnatal and in adult white matter as well as in oligodendrocyte cultures. Reverse transcription-PCR and Western blotting further confirmed the presence of CB1 receptors. Oligodendrocyte progenitors undergo apoptosis with the withdrawal of trophic support, as determined by TUNEL assay and caspase-3 activation, and both the selective CB1 agonist arachidonyl-2'-chloroethylamide/(all Z)-N-(2-cycloethyl)-5,8,11,14-eicosatetraenamide (ACEA) and the nonselective cannabinoid agonists HU210 and (+)-Win-55212-2 enhanced cell survival. To investigate intracellular signaling involved in cannabinoid protection, we focused on the phosphatidylinositol-3 kinase (PI3K)/Akt pathway. HU210, (+)-Win-55212-2, and ACEA elicited a time-dependent phosphorylation of Akt. Pertussis toxin abolished Akt activation, indicating the involvement of G(i)/G(o)-protein-coupled receptors. The CB1 receptor antagonist SR141716A partially inhibited Akt phosphorylation in response to HU210 and (+)-Win-55212-2 and abolished the effects of ACEA. Trophic support deprivation downregulated Akt activity, and cannabinoids recovered phospho-Akt levels. Inhibition of PI3K abrogated the survival action and the recovery of Akt activity in response to cannabinoids. SR141716A prevented only the protection conferred by ACEA. Nevertheless, SR141716A and the selective CB2 receptor antagonist SR144528 in combination inhibited the prosurvival action of HU210, which is in accordance with the finding of CB2 receptor expression by oligodendroglial cells. These data identify oligodendrocytes as potential targets of cannabinoid action in the CNS.
Figures




References
-
- Achiron A, Miron S, Lavie V, Margalit R, Biegon A. Dexanabinol (HU-211) effect on experimental autoimmune encephalomyelitis: implications for the treatment of acute relapses of multiple sclerosis. J Neuroimmunol. 2000;102:26–31. - PubMed
-
- Almazan G, Afar DEH, Bell JC. Phosphorylation and disruption of intermediate filament proteins in oligodendrocyte precursor cultures treated with calyculin A. J Neurosci Res. 1993;36:163–172. - PubMed
-
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
