Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus
- PMID: 12427843
- PMCID: PMC6757843
- DOI: 10.1523/JNEUROSCI.22-22-09868.2002
Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus
Abstract
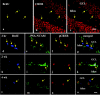
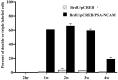
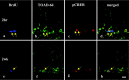
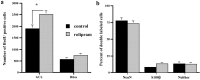
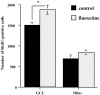
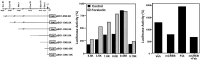
Neurogenesis continues to occur in the adult hippocampus, although many of the newborn cells degenerate 1-2 weeks after birth. The number and survival of newborn cells are regulated by a variety of environmental stimuli, but very little is known about the intracellular signal transduction pathways that control adult neurogenesis. In the present study, we examine the expression of the phosphorylated cAMP response element-binding protein (pCREB) in immature neurons in adult hippocampus and the role of the cAMP cascade in the survival of new neurons. The results demonstrate that virtually all immature neurons, identified by triple immunohistochemistry for bromodeoxyuridine (BrdU) and polysialic acid-neural cell adhesion molecule (PSA-NCAM), are also positive for pCREB. In addition, upregulation of cAMP (via pharmacological inhibition of cAMP breakdown or by antidepressant treatment) increases the survival of BrdU-positive cells. A possible role for pCREB in the regulation of PSA-NCAM, a marker of immature neurons involved in neuronal remodeling and neurite outgrowth, is supported by cell culture studies demonstrating that the cAMP-CREB pathway regulates the expression of a rate-limiting enzyme responsible for the synthesis of PSA-NCAM. These findings indicate that the cAMP-CREB pathway regulates the survival, and possibly the differentiation and function, of newborn neurons.
Figures

References
-
- Becker C, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996;45:143–152. - PubMed
-
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. - PubMed
-
- Bonfani L, Olive S, Poulain DA, Theodosis DT. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: an immunohistochemical study. J Neurosci. 1992;19:10228–10236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
