Thalamocortical bursts trigger recurrent activity in neocortical networks: layer 4 as a frequency-dependent gate
- PMID: 12427845
- PMCID: PMC6757840
- DOI: 10.1523/JNEUROSCI.22-22-09885.2002
Thalamocortical bursts trigger recurrent activity in neocortical networks: layer 4 as a frequency-dependent gate
Abstract
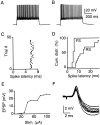
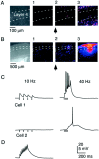
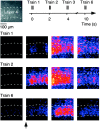
Sensory information reaches the cortex via thalamocortical (TC) synapses in layer 4. Thalamic relay neurons that mediate information flow to cortex operate in two distinct modes, tonic and burst firing. Burst firing has been implicated in enhancing reliability of information flow between individual neurons. However, little is known about how local networks of neocortical neurons respond to different temporal patterns of TC activity. We studied cortical activity patterns evoked by stimulating TC afferents at different frequencies, using a combination of electrophysiology and calcium imaging in TC slices that allowed for the reconstruction of spatiotemporal activity with single-cell resolution. Stimulation of TC axons at low frequencies triggered action potentials in only a small number of layer 4 neurons. In contrast, brief high-frequency stimulus trains triggered widespread recurrent activity in populations of neurons in layer 4 and then spread into adjacent layers 2/3 and 5. Recurrent activity had a clear threshold, typically lasted 300 msec, and could be evoked repetitively at frequencies up to 0.5 Hz. Moreover, the spatial extent of recurrent activity was controlled by the TC pattern of activity. Recurrent activity triggered within the highly interconnected networks of layer 4 might act to selectively amplify and redistribute transient high-frequency TC inputs, filter out low-frequency inputs, and temporarily preserve a record of past sensory activity.
Figures





References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol. 1994;341:39–49. - PubMed
-
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
