The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization
- PMID: 12428211
- PMCID: PMC378584
- DOI: 10.1086/344517
The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization
Abstract
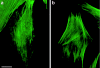
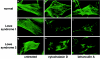
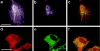
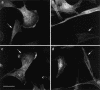
Lowe syndrome is a rare X-linked disorder characterized by bilateral congenital cataracts, renal Fanconi syndrome, and mental retardation. Lowe syndrome results from mutations in the OCRL1 gene, which encodes a phosphatidylinositol 4,5 bisphosphate 5-phosphatase located in the trans-Golgi network. As a first step in identifying the link between ocrl1 deficiency and the clinical disorder, we have identified a reproducible cellular abnormality of the actin cytoskeleton in fibroblasts from patients with Lowe syndrome. The cellular abnormality is characterized by a decrease in long actin stress fibers, enhanced sensitivity to actin depolymerizing agents, and an increase in punctate F-actin staining in a distinctly anomalous distribution in the center of the cell. We also demonstrate an abnormal distribution of two actin-binding proteins, gelsolin and alpha-actinin, proteins regulated by both PIP(2) and Ca(+2) that would be expected to be altered in Lowe cells. Actin polymerization plays a key role in the formation, maintenance, and proper function of tight junctions and adherens junctions, which have been demonstrated to be critical in renal proximal tubule function, and in the differentiation of the lens. These findings point to a general mechanism to explain how this PIP(2) 5-phosphatase deficiency might produce the Lowe syndrome phenotype.
Figures




References
Electronic-Database Information
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for OCRL [MIM 309000])
References
-
- Berridge MJ, Irvine RF (1989) Inositol phosphates and cell signaling. Nature 341:197–205 - PubMed
-
- Cereijido M, Gonzalez-Mariscal L, Contreras RG, Gallardo JM, Garciavillegas R, Valades J (1993) The making of a tight junction. J Cell Sci Suppl 17:127–132 - PubMed
-
- Dissmann E, Hinssen H (1994) Immunocytochemical localization of gelsolin in fibroblasts, myogenic cells, and isolated myofibrils. Eur J Cell Biol 63:336–344 - PubMed
-
- Dressman MA, Olivos-Glander IM, Nussbaum RL, Suchy SF (2000) Ocrl1, a Ptdlns(4,5)P2 5-phosphatase, is localized to the trans-Golgi network of fibroblasts and epithelial cells. J Histochem Cytochem 48:179–189 - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

