Epsilon-tubulin is an essential component of the centriole
- PMID: 12429830
- PMCID: PMC133598
- DOI: 10.1091/mbc.e02-04-0205
Epsilon-tubulin is an essential component of the centriole
Abstract
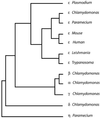
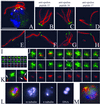
Centrioles and basal bodies are cylinders composed of nine triplet microtubule blades that play essential roles in the centrosome and in flagellar assembly. Chlamydomonas cells with the bld2-1 mutation fail to assemble doublet and triplet microtubules and have defects in cleavage furrow placement and meiosis. Using positional cloning, we have walked 720 kb and identified a 13.2-kb fragment that contains epsilon-tubulin and rescues the Bld2 defects. The bld2-1 allele has a premature stop codon and intragenic revertants replace the stop codon with glutamine, glutamate, or lysine. Polyclonal antibodies to epsilon-tubulin show peripheral labeling of full-length basal bodies and centrioles. Thus, epsilon-tubulin is encoded by the BLD2 allele and epsilon-tubulin plays a role in basal body/centriole morphogenesis.
Figures

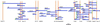



References
-
- Chang P, Stearns T. Delta-tubulin and epsilon-tubulin: two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nat Cell Biol. 2000;2:30–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

