The structural basis of pulmonary hypertension in chronic lung disease: remodelling, rarefaction or angiogenesis?
- PMID: 12430958
- PMCID: PMC1570922
- DOI: 10.1046/j.1469-7580.2002.00096.x
The structural basis of pulmonary hypertension in chronic lung disease: remodelling, rarefaction or angiogenesis?
Abstract
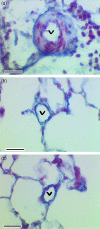
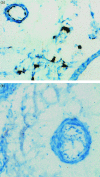
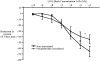
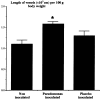
Chronic lung disease in humans is frequently complicated by the development of secondary pulmonary hypertension, which is associated with increased morbidity and mortality. Hypoxia, inflammation and increased shear stress are the primary stimuli although the exact pathways through which these initiating events lead to pulmonary hypertension remain to be completely elucidated. The increase in pulmonary vascular resistance is attributed, in part, to remodelling of the walls of resistance vessels. This consists of intimal, medial and adventitial hypertrophy, which can lead to encroachment into and reduction of the vascular lumen. In addition, it has been reported that there is a reduction in the number of blood vessels in the hypertensive lung, which could also contribute to increased vascular resistance. The pulmonary endothelium plays a key role in mediating and modulating these changes. These structural alterations in the pulmonary vasculature contrast sharply with the responses of the systemic vasculature to the same stimuli. In systemic organs, both hypoxia and inflammation cause angiogenesis. Furthermore, remodelling of the walls of resistance vessels is not observed in these conditions. Thus it has been generally stated that, in the adult pulmonary circulation, angiogenesis does not occur. Prompted by previous observations that chronic airway inflammation can lead to pulmonary vascular remodelling without hypertension, we have recently shown, using quantitative stereological techniques, that angiogenesis can occur in the adult pulmonary circulation. Pulmonary angiogenesis has also been reported in some other conditions including post-pneumonectomy lung growth, metastatic disease of the lung and in biliary cirrhosis. Such angiogenesis may serve to prevent or attenuate increased vascular resistance in lung disease. In view of these more recent data, the role of structural alterations in the pulmonary vasculature in the development of pulmonary hypertension should be carefully reconsidered.
Figures
References
-
- Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research ‘Work in progress’. Circulation. 2000;102:2781–2791. - PubMed
-
- Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int. Suppl. 1998;67:S100–S108. - PubMed
-
- Barbera JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, et al. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med. 1994;149:423–429. - PubMed
-
- Barbera JA, Peinado VI, Santos S, Ramirez J, Roca J, Rodriguez-Roisin R. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am. J. Respir. CritCare Med. 2001;164:709–713. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

