Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland
- PMID: 12432093
- PMCID: PMC137739
- DOI: 10.1073/pnas.242328999
Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland
Abstract
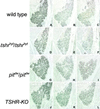
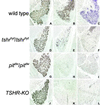
The thyroid-stimulating hormone/thyrotropin (TSH) is the most relevant hormone in the control of thyroid gland physiology in adulthood. TSH effects on the thyroid gland are mediated by the interaction with a specific TSH receptor (TSHR). We studied the role of TSHTSHR signaling on gland morphogenesis and differentiation in the mouse embryo using mouse lines deprived either of TSH (pit(dw)pit(dw)) or of a functional TSHR (tshr(hyt)tshr(hyt) and TSHR-knockout lines). The results reported here show that in the absence of either TSH or a functional TSHR, the thyroid gland develops to a normal size, whereas the expression of thyroperoxidase and the sodium/iodide symporter are reduced greatly. Conversely, no relevant changes are detected in the amounts of thyroglobulin and the thyroid-enriched transcription factors TTF-1, TTF-2, and Pax8. These data suggest that the major role of the TSH/TSHR pathway is in controlling genes involved in iodide metabolism such as sodium/iodide symporter and thyroperoxidase. Furthermore, our data indicate that in embryonic life TSH does not play an equivalent role in controlling gland growth as in the adult thyroid.
Figures





References
-
- Kaufman M. H. & Bard, J., (1999) The Anatomic Basis of Mouse Development (Academic, San Diego), pp. 165–166.
-
- Di Lauro R. & De Felice, M. (2001) in Endocrinology, eds. DeGroot, L. J. & Jameson, J. L. (Saunders, Philadelphia), Vol. 2, pp. 1268–1278.
-
- Lazzaro D., Price, M., De Felice, M. & Di Lauro, R. (1991) Development (Cambridge, U.K.) 113 1093-1104. - PubMed
-
- Plachov D., Chowdhury, K., Walther, C., Simon, D., Guenet, J. L. & Gruss, P. (1990) Development (Cambridge, U.K.) 110 643-651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

