Infection of a human hepatoma cell line by hepatitis B virus
- PMID: 12432097
- PMCID: PMC137772
- DOI: 10.1073/pnas.232137699
Infection of a human hepatoma cell line by hepatitis B virus
Abstract
Among numerous established human hepatoma cell lines, none has been shown susceptible to hepatitis B virus (HBV) infection. We describe here a cell line, called HepaRG, which exhibits hepatocyte-like morphology, expresses specific hepatocyte functions, and supports HBV infection as well as primary cultures of normal human hepatocytes. Differentiation and infectability are maintained only when these cells are cultured in the presence of corticoids and dimethyl sulfoxide. The specificity of this HBV infection model was ascertained by both the neutralization capacity of HBV-envelope protein-specific antibodies and the competition with an envelope-derived peptide. HepaRG cells therefore represent a tool for deciphering the mechanism of HBV entry. Moreover, their close resemblance to normal human hepatocytes makes them suitable for many applications including drug metabolism studies.
Figures
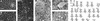
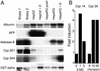
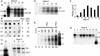
 ). (G) Southern blot kinetic analysis of extracellular HBV DNA in the supernatant of infected HepaRG cells. Complete viral particles were immunoprecipitated with an anti-HBsAg antibody from 1 ml of the supernatant of infected HepaRG cells. The viral DNA was extracted, and half of the preparation was analyzed by the Southern blot procedure. Lane M corresponds to 3.5 pg of 3.2- and 1.5-kb HBV DNA restriction fragments (3.4 and 6.8 × 106 molecules, respectively). Autoradiographic exposure time was 96 h.
). (G) Southern blot kinetic analysis of extracellular HBV DNA in the supernatant of infected HepaRG cells. Complete viral particles were immunoprecipitated with an anti-HBsAg antibody from 1 ml of the supernatant of infected HepaRG cells. The viral DNA was extracted, and half of the preparation was analyzed by the Southern blot procedure. Lane M corresponds to 3.5 pg of 3.2- and 1.5-kb HBV DNA restriction fragments (3.4 and 6.8 × 106 molecules, respectively). Autoradiographic exposure time was 96 h.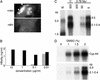
 ) or an unrelated monoclonal antibody (▪) and evaluated for their infectivity on HepaRG cells. Infectivity was evaluated by measuring the HBsAg secretion in the supernatant of infected cells at day 10 pi. (C) Peptide competition of HBV infection. HepaRG cells were preincubated with different concentrations (nM) of an HBV large protein-derived peptide (AA 2–78) bearing a myristic acid (myr) on gly 2 or, as a control, with a corresponding myristoylated duck hepatitis B virus-derived peptide (AA 2–41; C). The inoculum was then added for 20 h, without removing the peptide. Infectivity was evaluated by analyzing intracellular viral RNA at 10 days pi. Half of the RNA extracted from 106 infected cells was analyzed on a 1.5% agarose gel. Lane M corresponds to 3.5 pg of denatured 3.2- and 1.5-kb HBV DNA restriction fragments. Viral RNA sizes are indicated on the right. Autoradiographic exposure time was 48 h. (D) Efficiency of HBV infection and HepaRG cell differentiation. Northern blot analysis of CYP 3A4 (Upper) and HBV (Lower) RNAs after infection of HepaRG cells cultivated in presence of increasing concentrations of DMSO. RNA was extracted 10 days pi. Viral RNA sizes are indicated on the right.
) or an unrelated monoclonal antibody (▪) and evaluated for their infectivity on HepaRG cells. Infectivity was evaluated by measuring the HBsAg secretion in the supernatant of infected cells at day 10 pi. (C) Peptide competition of HBV infection. HepaRG cells were preincubated with different concentrations (nM) of an HBV large protein-derived peptide (AA 2–78) bearing a myristic acid (myr) on gly 2 or, as a control, with a corresponding myristoylated duck hepatitis B virus-derived peptide (AA 2–41; C). The inoculum was then added for 20 h, without removing the peptide. Infectivity was evaluated by analyzing intracellular viral RNA at 10 days pi. Half of the RNA extracted from 106 infected cells was analyzed on a 1.5% agarose gel. Lane M corresponds to 3.5 pg of denatured 3.2- and 1.5-kb HBV DNA restriction fragments. Viral RNA sizes are indicated on the right. Autoradiographic exposure time was 48 h. (D) Efficiency of HBV infection and HepaRG cell differentiation. Northern blot analysis of CYP 3A4 (Upper) and HBV (Lower) RNAs after infection of HepaRG cells cultivated in presence of increasing concentrations of DMSO. RNA was extracted 10 days pi. Viral RNA sizes are indicated on the right.References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

