Human, rat and chicken small intestinal Na+ - Cl- -creatine transporter: functional, molecular characterization and localization
- PMID: 12433955
- PMCID: PMC2290665
- DOI: 10.1113/jphysiol.2002.026377
Human, rat and chicken small intestinal Na+ - Cl- -creatine transporter: functional, molecular characterization and localization
Abstract
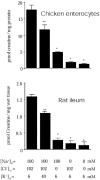
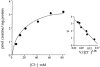
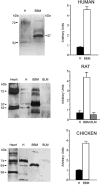
In spite of all the fascinating properties of oral creatine supplementation, the mechanism(s) mediating its intestinal absorption has(have) not been investigated. The purpose of this study was to characterize intestinal creatine transport. [(14)C] creatine uptake was measured in chicken enterocytes and rat ileum, and expression of the creatine transporter CRT was examined in human, rat and chicken small intestine by reverse transcription-polymerase chain reaction, Northern blot, in situ hybridization, immunoblotting and immunohistochemistry. Results show that enterocytes accumulate creatine against its concentration gradient. This accumulation was electrogenic, Na(+)- and Cl(-)-dependent, with a probable stoichiometry of 2 Na(+): 1 Cl(-): 1 creatine, and inhibited by ouabain and iodoacetic acid. The kinetic study revealed a K(m) for creatine of 29 microM. [(14)C] creatine uptake was efficiently antagonized by non-labelled creatine, guanidinopropionic acid and cyclocreatine. More distant structural analogues of creatine, such as GABA, choline, glycine, beta-alanine, taurine and betaine, had no effect on intestinal creatine uptake, indicating a high substrate specificity of the creatine transporter. Consistent with these functional data, messenger RNA for CRT was detected only in the cells lining the intestinal villus. The sequences of partial clones, and of the full-length cDNA clone, isolated from human and rat small intestine were identical to previously cloned CRT cDNAs. Immunological analysis revealed that CRT protein was mainly associated with the apical membrane of the enterocytes. This study reports for the first time that mammalian and avian enterocytes express CRT along the villus, where it mediates high-affinity, Na(+)- and Cl(-)-dependent, apical creatine uptake.
Figures






References
-
- Barnett C, Hinds M, Jenkins D. Effects of oral creatine supplementation on multiple sprint cycle performance. Australian Journal of Science and Medicine in Sport. 1996;28:35–39. - PubMed
-
- Bessman SP, Carpenter CL. The creatine-creatine phosphate energy shuttle. Annual Review of Biochemistry. 1985;54:831–862. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72:248–254. - PubMed
-
- Braissant O, Henry H, Loup M, Eilers B, Bachmann C. Endogenous synthesis and transport of creatine in the rat brain, an in situ hybridization study. Molecular Brain Research. 2001;86:193–201. - PubMed
-
- Burke L, Pyne D, Telford R. Effect of oral creatine supplementation on single-effort sprint performance in elite swimmers. International Journal of Sport Nutrition. 1996;6:222–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

