Secretion and cell volume regulation by salivary acinar cells from mice lacking expression of the Clcn3 Cl- channel gene
- PMID: 12433961
- PMCID: PMC2290643
- DOI: 10.1113/jphysiol.2002.021980
Secretion and cell volume regulation by salivary acinar cells from mice lacking expression of the Clcn3 Cl- channel gene
Abstract
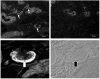
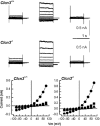
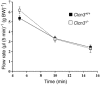
Salivary gland acinar cells shrink when Cl(-) currents are activated following cell swelling induced by exposure to a hypotonic solution or in response to calcium-mobilizing agonists. The molecular identity of the Cl(-) channel(s) in salivary cells involved in these processes is unknown, although ClC-3 has been implicated in several tissues as a cell-volume-sensitive Cl(-) channel. We found that cells isolated from mice with targeted disruption of the Clcn3 gene undergo regulatory volume decrease in a fashion similar to cells from wild-type littermates. Consistent with a normal regulatory volume decrease response, the magnitude and the kinetics of the swell-activated Cl(-) currents in cells from ClC-3-deficient mice were equivalent to those from wild-type mice. It has also been suggested that ClC-3 is activated by Ca(2+)-calmodulin-dependent protein kinase II; however, the magnitude of the Ca(2+)-dependent Cl(-) current was unchanged in the Clcn3(-/-) animals. In addition, we observed that ClC-3 appeared to be highly expressed in the smooth muscle cells of glandular blood vessels, suggesting a potential role for this channel in saliva production by regulating blood flow, yet the volume and ionic compositions of in vivo stimulated saliva from wild-type and null mutant animals were comparable. Finally, in some cells ClC-3 is an intracellular channel that is thought to be involved in vesicular acidification and secretion. Nevertheless, the protein content of saliva was unchanged in Clcn3(-/-) mice. Our results demonstrate that the ClC-3 Cl(-) channel is not a major regulator of acinar cell volume, nor is it essential for determining the secretion rate and composition of saliva.
Figures



References
-
- Begenisich T, Melvin JE. Regulation of chloride channels in secretory epithelia. Journal of Membrane Biology. 1998;163:77–85. - PubMed
-
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods in Cell Biology. 1994;40:3–29. - PubMed
-
- Castle JD, Cameron RS, Arvan P, Von Zastrow M, Rudnick G. Similarities and differences among neuroendocrine, exocrine, and endocytic vesicles. Annals of the New York Academy of Sciences. 1987;493:448–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

