Chemical chaperones increase the cellular activity of N370S beta -glucosidase: a therapeutic strategy for Gaucher disease
- PMID: 12434014
- PMCID: PMC137733
- DOI: 10.1073/pnas.192582899
Chemical chaperones increase the cellular activity of N370S beta -glucosidase: a therapeutic strategy for Gaucher disease
Abstract
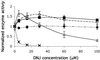
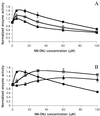
Gaucher disease is a lysosomal storage disorder caused by deficient lysosomal beta-glucosidase (beta-Glu) activity. A marked decrease in enzyme activity results in progressive accumulation of the substrate (glucosylceramide) in macrophages, leading to hepatosplenomegaly, anemia, skeletal lesions, and sometimes CNS involvement. Enzyme replacement therapy for Gaucher disease is costly and relatively ineffective for CNS involvement. Chemical chaperones have been shown to stabilize various proteins against misfolding, increasing proper trafficking from the endoplasmic reticulum. We report herein that the addition of subinhibitory concentrations (10 microM) of N-(n-nonyl)deoxynojirimycin (NN-DNJ) to a fibroblast culture medium for 9 days leads to a 2-fold increase in the activity of N370S beta-Glu, the most common mutation causing Gaucher disease. Moreover, the increased activity persists for at least 6 days after the withdrawal of the putative chaperone. The NN-DNJ chaperone also increases WT beta-Glu activity, but not that of L444P, a less prevalent Gaucher disease variant. Incubation of isolated soluble WT enzyme with NN-DNJ reveals that beta-Glu is stabilized against heat denaturation in a dose-dependent fashion. We propose that NN-DNJ chaperones beta-Glu folding at neutral pH, thus allowing the stabilized enzyme to transit from the endoplasmic reticulum to the Golgi, enabling proper trafficking to the lysosome. Clinical data suggest that a modest increase in beta-Glu activity may be sufficient to achieve a therapeutic effect.
Figures


References
-
- Winchester B., Vellodi, A. & Young, E. (2000) Biochem. Soc. Trans. 28 150-154. - PubMed
-
- Rapola J. (1994) Pathol. Res. Pract. 190 759-766. - PubMed
-
- Durand P., Fabrega, S., Henrissat, B., Mornon, J.-P. & Lehn, P. (2000) Hum. Mol. Genet. 9 967-977. - PubMed
-
- Schiffmann R. & Brady, R. O. (2002) Drugs 62 733-742. - PubMed
-
- Bijsterbosch M. K., Donker, W., van de Bilt, H., van Weely, S., van Berkel, T. J. & Aerts, J. M. (1996) Eur. J. Biochem. 237 344-349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

