Drug susceptibilities of yeast cells are affected by membrane lipid composition
- PMID: 12435664
- PMCID: PMC132749
- DOI: 10.1128/AAC.46.12.3695-3705.2002
Drug susceptibilities of yeast cells are affected by membrane lipid composition
Abstract
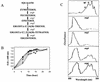
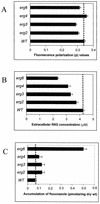
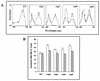
In the present study we have exploited isogenic erg mutants of Saccharomyces cerevisiae to examine the contribution of an altered lipid environment on drug susceptibilities of yeast cells. It is observed that erg mutants, which possess high levels of membrane fluidity, were hypersensitive to the drugs tested, i.e., cycloheximide (CYH), o-phenanthroline, sulfomethuron methyl, 4-nitroquinoline oxide, and methotrexate. Most of the erg mutants except mutant erg4 were, however, resistant to fluconazole (FLC). By using the fluorophore rhodamine-6G and radiolabeled FLC to monitor the passive diffusion, it was observed that erg mutant cells elicited enhanced diffusion. The addition of a membrane fluidizer, benzyl alcohol (BA), to S. cerevisiae wild-type cells led to enhanced membrane fluidity. However, a 10 to 12% increase in BA-induced membrane fluidity did not alter the drug susceptibilities of the S. cerevisiae wild-type cells. The enhanced diffusion observed in erg mutants did not seem to be solely responsible for the observed hypersensitivity of erg mutants. In order to ascertain the functioning of drug extrusion pumps encoding the genes CDR1 (ATP-binding cassette family) and CaMDR1 (MFS family) of Candida albicans in a different lipid environment, they were independently expressed in an S. cerevisiae erg mutant background. While the fold change in drug resistance mediated by CaMDR1 remained the same or increased in erg mutants, susceptibility to FLC and CYH mediated by CDR1 was increased (decrease in fold resistance). Our results demonstrate that between the two drug extrusion pumps, Cdr1p appeared to be more adversely affected by the fluctuations in the membrane lipid environment (particularly to ergosterol). By using 6-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino-hexanoyl] sphingosyl phosphocholine (a fluorescent analogue of sphingomyelin), a close interaction between membrane ergosterol and sphingomyelin which appears to be disrupted in erg mutants is demonstrated. Taken together it appears that multidrug resistance in yeast is closely linked to the status of membrane lipids, wherein the overall drug susceptibility phenotype of a cell appears to be an interplay among drug diffusion, extrusion pumps, and the membrane lipid environment.
Figures




References
-
- Ansari, S., P. Gupta, S. K. Mahanty, and R. Prasad. 1993. The uptake of amino acids by erg mutants of Candida albicans. J. Med. Vet. Mycol. 31:377-386.
-
- Arthington-Skaggs, B., W. Lee-Yang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 46:2477-2481. - PMC - PubMed
-
- Debry, P., E. A. Nash, D. W. Nekalson, and J. E. Metherall. 1997. Role of multidrug resistance P-glycoproteins in cholesterol esterification. J. Biol. Chem. 272:1026-1031. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

