Target site concentrations of ciprofloxacin after single intravenous and oral doses
- PMID: 12435668
- PMCID: PMC132760
- DOI: 10.1128/AAC.46.12.3724-3730.2002
Target site concentrations of ciprofloxacin after single intravenous and oral doses
Abstract
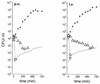
To characterize the potential of ciprofloxacin penetration into human soft tissues following intravenous (i.v.) and oral (p.o.) administration, we measured the free ciprofloxacin concentrations in interstitial space fluid of skeletal muscle and subcutaneous adipose tissue by microdialysis. In addition, ciprofloxacin concentrations were measured in cantharis-induced skin blisters, saliva, and capillary plasma and were compared to the total concentrations in venous plasma. Furthermore, a pharmacodynamic in vitro model was used to simulate in vivo pharmacokinetics in bacterial culture. Eight healthy volunteers received ciprofloxacin in an open randomized crossover fashion either as a single i.v. infusion of 400 mg over 60 min or as a single p.o. dose of 500 mg. For both tissues the mean areas under the concentration-time curves (AUCs) for interstitial space fluid (AUC(interstitial fluid)s) were significantly lower than the corresponding AUC(plasma)s, with AUC(interstitial fluid)/AUC(plasma) ratios ranging from 0.38 to 0.68. For skeletal muscle, the AUC(interstitial fluid) was significantly higher after administration of 400 mg i.v. than after administration of 500 mg p.o., with a ratio of the AUC after p.o. administration/AUC after i.v. administration of 0.64. The ratio of the concentration in skeletal muscle/concentration in plasma increased over the entire observation period, implying that ciprofloxacin concentrations were not at steady state. The ratio of the concentration in skin blister fluid/concentration in plasma reached values above 4, indicating a preferential penetration of ciprofloxacin into inflamed lesions. The concentrations in saliva and capillary blood were similar to the corresponding total levels in plasma. In vitro both in vivo ciprofloxacin concentration-time profiles were equally effective against select bacterial strains. In conclusion, single-dose administration of two bioequivalent dosage forms of ciprofloxacin might lead to differences in target site pharmacokinetics. These differences, however, are not related to a difference in target site pharmacodynamics.
Figures



References
-
- Amodio-Groton, M., A. Madu, C. N. Madu, L. L. Briceland, M. Seligman, P. McMaster, and M. H. Miller. 1996. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: a pharmacoeconomic analysis. Ann. Pharmacother. 30:596-602. - PubMed
-
- Bannefeld, K. H., H. Stass, and G. Blaschke. 1997. Capillary electrophoresis with laser-induced fluorescence detection, an adequate alternative to high performance liquid chromatography, for the determination of ciprofloxacin and its metabolite desethyleneciprofloxacin in human plasma. J. Chromatogr. B 692:453-459. - PubMed
-
- Carlier, M. B., B. Scorneaux, A. Zenebergh, J. F. Desnottes, and P. M. Tulkens. 1990. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J. Antimicrob. Chemother. 26(Suppl. B):27-39. - PubMed
-
- Davis, R., A. Markham, and J. A. Balfour. 1996. Ciprofloxacin. An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs 51:1019-1074. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

