Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists
- PMID: 12437772
- PMCID: PMC137065
- DOI: 10.1186/1475-4924-1-10
Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists
Abstract
Background: The Hedgehog (Hh) signaling pathway is vital to animal development as it mediates the differentiation of multiple cell types during embryogenesis. In adults, Hh signaling can be activated to facilitate tissue maintenance and repair. Moreover, stimulation of the Hh pathway has shown therapeutic efficacy in models of neuropathy. The underlying mechanisms of Hh signal transduction remain obscure, however: little is known about the communication between the pathway suppressor Patched (Ptc), a multipass transmembrane protein that directly binds Hh, and the pathway activator Smoothened (Smo), a protein that is related to G-protein-coupled receptors and is capable of constitutive activation in the absence of Ptc.
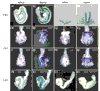
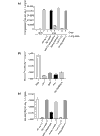
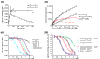
Results: We have identified and characterized a synthetic non-peptidyl small molecule, Hh-Ag, that acts as an agonist of the Hh pathway. This Hh agonist promotes cell-type-specific proliferation and concentration-dependent differentiation in vitro, while in utero it rescues aspects of the Hh-signaling defect in Sonic hedgehog-null, but not Smo-null, mouse embryos. Biochemical studies with Hh-Ag, the Hh-signaling antagonist cyclopamine, and a novel Hh-signaling inhibitor Cur61414, reveal that the action of all these compounds is independent of Hh-protein ligand and of the Hh receptor Ptc, as each binds directly to Smo.
Conclusions: Smo can have its activity modulated directly by synthetic small molecules. These studies raise the possibility that Hh signaling may be regulated by endogenous small molecules in vivo and provide potent compounds with which to test the therapeutic value of activating the Hh-signaling pathway in the treatment of traumatic and chronic degenerative conditions.
Figures





References
-
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. - PubMed
-
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. - PubMed
-
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. - PubMed
-
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. - PubMed
-
- Denef N, Neubuser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–531. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

