A glycoconjugate vaccine for Neisseria meningitidis induces antibodies in human infants that afford protection against meningococcal bacteremia in a neonate rat challenge model
- PMID: 12438327
- PMCID: PMC133014
- DOI: 10.1128/IAI.70.12.6576-6582.2002
A glycoconjugate vaccine for Neisseria meningitidis induces antibodies in human infants that afford protection against meningococcal bacteremia in a neonate rat challenge model
Abstract
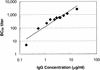
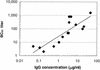
The functional activities of serum samples from human infants immunized with a glycoconjugate vaccine for Neisseria meningitidis serogroup C were assessed in a complement-mediated antibody-dependent serum bactericidal assay (SBA) and in a neonate rat model of protection from bacteremia. Selective serum samples from individual human infants were combined to make a panel of 11 serum pools to obtain a sufficient volume for testing. Each pool was assayed (i) for the anti-N. meningitidis serogroup C capsular polysaccharide (PS) immunoglobulin G (IgG) concentration as determined by reactivity in a direct-binding enzyme-linked immunosorbent assay, (ii) for bactericidal activity against N. meningitidis serogroup C strain C11, and (iii) for the ability to reduce bacteremia after passive transfer into a neonate rat model. Representative serum samples from infants who were not previously immunized with any N. meningitidis serogroup C vaccine served as a negative control. The prepared serum pools ranged in antibody concentration from 0.18 to 17.31 micro g of IgG specific for N. meningitidis serogroup C PS per ml. For this serum panel, a direct relationship between concentrations of anti-N. meningitidis serogroup C PS-specific IgG and serum SBA titers (r = 0.9960) was observed. Passive transfer to neonate rats demonstrated the ability of postimmunization serum samples to significantly reduce (> or =2-log(10) reduction compared to control animals) the level of bacteremia following a challenge. Of 79 neonate rats that received > or =0.031 micro g of human infant anti-N. meningitidis serogroup C PS IgG, 75 (94.9%) had a > or =2-log(10) reduction in bacteremia, whereas of the animals that received <0.031 micro g of antigen-specific IgG, 10.3% (4 of 39 rats) showed a > or =2-log(10) reduction in bacteremia. It was concluded that the anti-N. meningitidis serogroup C PS IgG antibody induced by this glycoconjugate vaccine had in vitro functional activity (as determined by a SBA) and also afforded protection against meningococcal bacteremia in an animal model.
Figures
Similar articles
-
Age-related disparity in functional activities of human group C serum anticapsular antibodies elicited by meningococcal polysaccharide vaccine.Infect Immun. 2003 Jan;71(1):275-86. doi: 10.1128/IAI.71.1.275-286.2003. Infect Immun. 2003. PMID: 12496177 Free PMC article.
-
Relationship between serum bactericidal activity and serogroup-specific immunoglobulin G concentration for adults, toddlers, and infants immunized with Neisseria meningitidis serogroup C vaccines.Clin Diagn Lab Immunol. 2000 Sep;7(5):764-8. doi: 10.1128/CDLI.7.5.764-768.2000. Clin Diagn Lab Immunol. 2000. PMID: 10973451 Free PMC article. Clinical Trial.
-
Persistence of serum bactericidal antibody one year after a booster dose of either a glycoconjugate or a plain polysaccharide vaccine against serogroup C Neisseria meningitidis given to adolescents previously immunized with a glycoconjugate vaccine.Pediatr Infect Dis J. 2011 Nov;30(11):e203-8. doi: 10.1097/INF.0b013e318224fb14. Pediatr Infect Dis J. 2011. PMID: 21673612 Clinical Trial.
-
Quadrivalent meningococcal ACYW-135 glycoconjugate vaccine for broader protection from infancy.Expert Rev Vaccines. 2009 May;8(5):529-42. doi: 10.1586/erv.09.18. Expert Rev Vaccines. 2009. PMID: 19397410 Review.
-
Immunogenicity and safety of a meningococcal serogroup A, C, Y and W glycoconjugate vaccine, ACWY-TT.Adv Ther. 2013 May;30(5):431-58. doi: 10.1007/s12325-013-0032-5. Epub 2013 May 28. Adv Ther. 2013. PMID: 23712402 Review.
Cited by
-
Evaluation of recombinant lipidated P2086 protein as a vaccine candidate for group B Neisseria meningitidis in a murine nasal challenge model.Infect Immun. 2005 Oct;73(10):6838-45. doi: 10.1128/IAI.73.10.6838-6845.2005. Infect Immun. 2005. PMID: 16177362 Free PMC article.
References
-
- Andreoni, J., H. Kayhty, and P. Densen. 1993. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J. Infect. Dis. 168:227-231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

