Leishmania pifanoi pathogenesis: selective lack of a local cutaneous response in the absence of circulating antibody
- PMID: 12438331
- PMCID: PMC132956
- DOI: 10.1128/IAI.70.12.6597-6605.2002
Leishmania pifanoi pathogenesis: selective lack of a local cutaneous response in the absence of circulating antibody
Abstract
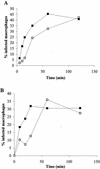
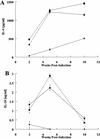
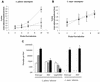
Recently, a role for B cells in the pathogenesis associated with infection by Leishmania (Leishmania mexicana complex and L. donovani) has been established. In the case of L. mexicana complex parasites (L. mexicana, L. pifanoi, and L. amazonensis), a critical role for immunoglobulin G-mediated mechanisms for the amastigote stage in the host is evident; however, the immunological mechanisms involved remain to be established. In vitro analysis of the kinetics of parasite uptake by macrophages failed to indicate a major effect of antibody opsonization. Given the importance of CD4(+) T cells in the development of disease caused by these parasites, the possibility that the lack of pathogenesis was due to the lack of development of an immune response at the local site (draining lymph node and/or cutaneous site) was explored. Interestingly, the level of CD4(+)-T-cell activation (proliferation and cytokine) in draining lymph nodes from mice lacking circulating antibody (resistant) was found to be comparable to that in nodes from wild-type mice (susceptible) at 2, 5, and 10 weeks postinfection. However, antibody-deficient animals had markedly reduced numbers of monocytes and lymphocytes recruited or retained at the site of cutaneous infection in comparison to wild-type mice, indicating a selective impairment in the local cutaneous immune response. In vitro antigen presentation studies employing tissue-derived (opsonized) amastigotes demonstrated that L. pifanoi-infected FcR(-/-) macrophages, in contrast to comparably infected wild-type cells, failed to activate Leishmania antigen-specific T lymphocytes. These data, taken together, suggest that one possible mechanism for the role of antibody in pathogenesis may be to mediate parasite uptake and regulate the immune response at the local cutaneous site of infection.
Figures



References
-
- Alonso, A., Y. Bayon, M. Renedo, and M. S. Crespo. 2000. Stimulation of Fc gamma R receptors induces monocyte chemoattractant protein-1 in the human monocytic cell line THP-1 by a mechanism involving I kappa B-alpha degradation and formation of p50/p65 NF-kappa B/Rel complexes. Int. Immunol. 12:547-554. - PubMed
-
- Balestieri, F. M., A. R. Queiroz, C. Scavone, V. M. Costa, M. Barral-Netto, and A. Abrahamsohn Ide. 2002. Leishmania (L.) amazonensis-induced inhibition of nitric oxide synthesis in host macrophages. Microbes Infect. 4:23-29. - PubMed
-
- Barral-Netto, M., A. Barral, C. E. Brownell, Y. A. Skeiky, L. R. Ellingsworth, D. R. Twardzik, and S. G. Reed. 1992. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257:545-548. - PubMed
-
- Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

