EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis
- PMID: 12438337
- PMCID: PMC133027
- DOI: 10.1128/IAI.70.12.6646-6651.2002
EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis
Abstract
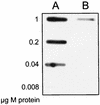
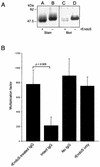
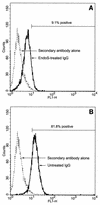
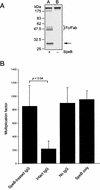
The human pathogen Streptococcus pyogenes primarily infects the upper respiratory tract and skin, but occasionally it disseminates and causes severe invasive disease with high mortality. This study revealed that the activity of extracellular EndoS, which hydrolyzes the functionally important N-linked oligosaccharides on opsonizing immunoglobulin G (IgG), contributes to increased survival of S. pyogenes in human blood ex vivo. The inability to kill the bacteria is due to reduced binding of IgG to Fc receptors and impaired classical pathway-mediated activation of complement. In addition, the activity of extracellular SpeB, which cleaves IgG into Fc and Fab fragments, also increases bacterial survival. This suggests that S. pyogenes expresses two enzymes, EndoS and SpeB, which modulate IgG by different mechanisms in order to evade the adaptive immune system.
Figures

References
-
- Åkesson, P., J. Cooney, F. Kishimoto, and L. Björck. 1990. Protein H—a novel IgG binding bacterial protein. Mol. Immunol. 27:523-531. - PubMed
-
- Berge, A., and L. Björck. 1995. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 270:9862-9867. - PubMed
-
- Chuang, P. D., and S. L. Morrison. 1997. Elimination of N-linked glycosylation sites from the human IgA1 constant region: effects on structure and function. J. Immunol. 158:724-732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

