Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges
- PMID: 12438346
- PMCID: PMC132946
- DOI: 10.1128/IAI.70.12.6715-6725.2002
Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges
Abstract
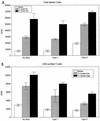
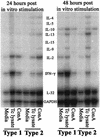
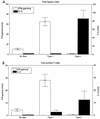
Chagas' disease results from infection with Trypanosoma cruzi, a protozoan parasite that establishes systemic intracellular infection after mucosal invasion. We hypothesized that ideal vaccines for mucosally invasive, intracellular pathogens like T. cruzi should induce mucosal type 2 immunity for optimal induction of protective secretory immunoglobulin A (IgA) and systemic type 1 immunity protective against intracellular replication. However, differential mucosal and systemic immune memory could be difficult to induce because of reciprocal inhibitory actions between type 1 and type 2 responses. To test our hypotheses, we investigated the protective effects of type 1 and type 2 biased vaccines against mucosal and systemic T. cruzi challenges. Intranasal vaccinations were given with recombinant interleukin-12 (IL-12)- and IL-4-neutralizing antibody (Ab) for type 1 immune bias, or recombinant IL-4 and gamma interferon-neutralizing Ab for type 2 immune bias. Cytokine RNA and protein studies confirmed that highly polarized memory immune responses were induced by our vaccination protocols. Survival after virulent subcutaneous T. cruzi challenge was used to assess systemic protection. Mucosal protection was assessed by measuring the relative inhibition of parasite replication in mucosal tissues early after oral T. cruzi challenge, using both PCR and quantitative culture techniques. As expected, only type 1 responses protected against systemic challenges (P < 0.01). However, contrary to our original hypothesis, type 1 responses optimally protected against mucosal challenges as well (P < 0.05). Type 1 and type 2 biased vaccines induced similar secretory IgA responses. We conclude that future vaccines for T. cruzi and possibly other mucosally invasive, intracellular pathogens should induce both mucosal and systemic type 1 immunity.
Figures




Similar articles
-
Intranasal vaccinations with the trans-sialidase antigen plus CpG Adjuvant induce mucosal immunity protective against conjunctival Trypanosoma cruzi challenges.Infect Immun. 2010 Mar;78(3):1333-8. doi: 10.1128/IAI.00278-09. Epub 2010 Jan 4. Infect Immun. 2010. PMID: 20048046 Free PMC article.
-
Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice.Infect Immun. 2002 Sep;70(9):5065-74. doi: 10.1128/IAI.70.9.5065-5074.2002. Infect Immun. 2002. PMID: 12183554 Free PMC article.
-
Involvement of CD4(+) Th1 cells in systemic immunity protective against primary and secondary challenges with Trypanosoma cruzi.Infect Immun. 2000 Jan;68(1):197-204. doi: 10.1128/IAI.68.1.197-204.2000. Infect Immun. 2000. PMID: 10603388 Free PMC article.
-
Interleukin 12 and innate molecules for enhanced mucosal immunity.Immunol Res. 1999;20(3):207-17. doi: 10.1007/BF02790404. Immunol Res. 1999. PMID: 10741861 Review.
-
Vaccines and the regulatory arm of the immune system. An overview from the Trypanosoma cruzi infection model.Vaccine. 2019 Jun 19;37(28):3628-3637. doi: 10.1016/j.vaccine.2019.05.015. Epub 2019 May 31. Vaccine. 2019. PMID: 31155420 Review.
Cited by
-
Trypanosoma cruzi Entrance through Systemic or Mucosal Infection Sites Differentially Modulates Regional Immune Response Following Acute Infection in Mice.Front Immunol. 2013 Jul 26;4:216. doi: 10.3389/fimmu.2013.00216. eCollection 2013. Front Immunol. 2013. PMID: 23898334 Free PMC article.
-
Type 1 immunity provides both optimal mucosal and systemic protection against a mucosally invasive, intracellular pathogen.Infect Immun. 2005 Aug;73(8):4934-40. doi: 10.1128/IAI.73.8.4934-4940.2005. Infect Immun. 2005. PMID: 16041007 Free PMC article.
-
Specific humoral and cellular immunity induced by Trypanosoma cruzi DNA immunization in a canine model.Vet Res. 2013 Mar 11;44(1):15. doi: 10.1186/1297-9716-44-15. Vet Res. 2013. PMID: 23497041 Free PMC article.
-
Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice.Infect Immun. 2004 Jan;72(1):46-53. doi: 10.1128/IAI.72.1.46-53.2004. Infect Immun. 2004. PMID: 14688079 Free PMC article.
-
A terpenoid-rich extract from Clethra fimbriata exhibits anti-Trypanosoma cru zi activity and induces T cell cytokine production.Heliyon. 2022 Mar 25;8(3):e09182. doi: 10.1016/j.heliyon.2022.e09182. eCollection 2022 Mar. Heliyon. 2022. PMID: 35368545 Free PMC article.
References
-
- Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. - PubMed
-
- Andersen, P. 2001. TB vaccines: progress and problems. Trends Immunol. 22:160-168. - PubMed
-
- Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis. 180:940-949. - PubMed
-
- Boyaka, P. N., M. Marinaro, R. J. Jackson, S. Menon, H. Kiyono, E. Jirillo, and J. R. McGhee. 1999. IL-12 is an effective adjuvant for induction of mucosal immunity. J. Immunol. 162:122-128. - PubMed
-
- Calvo Mendez, M. L., B. Nogueda Torres, and R. Alejandre Aguilar. 1992. La via oral: una puerta de acceso para Trypanosoma cruzi. Rev. Latinoam. Microbiol. 34:39-42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

