Activation of myosin V-based motility and F-actin-dependent network formation of endoplasmic reticulum during mitosis
- PMID: 12438410
- PMCID: PMC2173107
- DOI: 10.1083/jcb.200204065
Activation of myosin V-based motility and F-actin-dependent network formation of endoplasmic reticulum during mitosis
Abstract
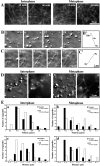
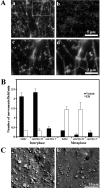
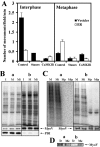
It is widely believed that microtubule- and F-actin-based transport of cytoplasmic organelles and membrane fusion is down-regulated during mitosis. Here we show that during the transition of Xenopus egg extracts from interphase to metaphase myosin V-driven movement of small globular vesicles along F-actin is strongly inhibited. In contrast, the movement of ER and ER network formation on F-actin is up-regulated in metaphase extracts. Our data demonstrate that myosin V-driven motility of distinct organelles is differently controlled during the cell cycle and suggest an active role of F-actin in partitioning, positioning, and membrane fusion of the ER during cell division.
Figures


Similar articles
-
Cell cycle regulation of myosin-V by calcium/calmodulin-dependent protein kinase II.Science. 2001 Aug 17;293(5533):1317-20. doi: 10.1126/science.1061086. Science. 2001. PMID: 11509731
-
Regulation of melanosome movement in the cell cycle by reversible association with myosin V.J Cell Biol. 1999 Sep 20;146(6):1265-76. doi: 10.1083/jcb.146.6.1265. J Cell Biol. 1999. PMID: 10491390 Free PMC article.
-
Microinjected F-actin into dividing newt eggs moves toward the next cleavage furrow together with Ca2+ stores with inositol 1,4,5-trisphosphate receptor in a microtubule- and microtubule motor-dependent manner.Ital J Anat Embryol. 2008 Jul-Sep;113(3):143-51. Ital J Anat Embryol. 2008. PMID: 19205586
-
Dynamics and inheritance of the endoplasmic reticulum.J Cell Sci. 2004 Jun 15;117(Pt 14):2871-8. doi: 10.1242/jcs.01286. J Cell Sci. 2004. PMID: 15197242 Review.
-
Vesicle transport: the role of actin filaments and myosin motors.Microsc Res Tech. 1999 Oct 15;47(2):93-106. doi: 10.1002/(SICI)1097-0029(19991015)47:2<93::AID-JEMT2>3.0.CO;2-P. Microsc Res Tech. 1999. PMID: 10523788 Review.
Cited by
-
Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells.J Cell Biol. 2007 Dec 3;179(5):895-909. doi: 10.1083/jcb.200705112. J Cell Biol. 2007. PMID: 18056408 Free PMC article.
-
A tubule-sheet continuum model for the mechanism of nuclear envelope assembly.Dev Cell. 2023 May 22;58(10):847-865.e10. doi: 10.1016/j.devcel.2023.04.003. Epub 2023 Apr 24. Dev Cell. 2023. PMID: 37098350 Free PMC article.
-
Intertwined and Finely Balanced: Endoplasmic Reticulum Morphology, Dynamics, Function, and Diseases.Cells. 2021 Sep 7;10(9):2341. doi: 10.3390/cells10092341. Cells. 2021. PMID: 34571990 Free PMC article. Review.
-
Myosin Va participates in acrosomal formation and nuclear morphogenesis during spermatogenesis of Chinese mitten crab Eriocheir sinensis.PLoS One. 2010 Sep 14;5(9):e12738. doi: 10.1371/journal.pone.0012738. PLoS One. 2010. PMID: 20856877 Free PMC article.
-
Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of alzheimer's disease.Aging Cell. 2003 Dec;2(6):305-18. doi: 10.1046/j.1474-9728.2003.00069.x. Aging Cell. 2003. PMID: 14677633 Free PMC article.
References
-
- Felix, M.A., P.R. Clarke, J. Verde, and E. Karsenti. 1993. Xenopus egg extracts as a system for studing mitosis. The Cell Cycle—A Practical Approach. P. Fantes and R. Brooks, editors. Oxford University Press Inc., New York. 253–283.
-
- Glotzer, M., A.W. Murray, and M.W. Kirschner. 1991. Cyclin is degraded by the ubiquitin pathway. Nature. 349:132–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

