Recipient iNOS but not eNOS deficiency reduces luminal narrowing in tracheal allografts
- PMID: 12438423
- PMCID: PMC2193988
- DOI: 10.1084/jem.20012135
Recipient iNOS but not eNOS deficiency reduces luminal narrowing in tracheal allografts
Abstract
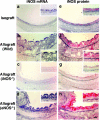
Chronic airway rejection is characterized by prolonged inflammation, epithelial damage, and eventual luminal obliterative bronchiolitis (OB). In cardiac allografts, the inducible nitric oxide synthase (iNOS) promotes acute rejection but paradoxically reduces neointimal formation, the hallmark of chronic rejection. The specific roles of NOS isoforms in modulating lymphocyte traffic and airway rejection are not known. Using a double lumen mouse tracheal transplant model, tracheal grafts from B10.A (allo) or C57BL/6J (iso) mice were transplanted into cyclosporine-treated wild-type (WT) iNOS(-/-) or endothelial NOS (eNOS)(-/-) recipients. OB was observed in WT tracheal allografts at 3 weeks (53 +/- 2% luminal occlusion vs. 17 +/- 1% for isografts, P < 0.05) with sites of obstructive lesion formation coinciding with areas of CD3(+) CD8(+) T cell-rich lymphocytic bronchitis. In contrast, allografts in iNOS(-/-) recipients exhibited reductions in local expression of proinflammatory chemokines and cytokines, graft T cell recruitment and apoptosis, and luminal obliteration (29 +/- 2%, P < 0.05 vs. WT allografts). Recipient eNOS deficiency, however, suppressed neither chemokine expression, lymphocyte infiltration, nor airway occlusion (54 +/- 2%). These data demonstrate that iNOS exacerbates luminal obliteration of airway allografts in contrast with the known suppression by iNOS of cardiac allograft vasculopathy. Because iNOS(-/-) airways transplanted into WT allograft hosts are not protected from rejection, these data suggest that iNOS expressed by graft-infiltrating leukocytes exerts the dominant influence on airway rejection.
Figures












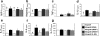
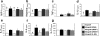
Similar articles
-
Early growth response gene-1 promotes airway allograft rejection.Am J Physiol Lung Cell Mol Physiol. 2007 Jul;293(1):L124-30. doi: 10.1152/ajplung.00285.2006. Epub 2007 Mar 23. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17384085
-
Endothelial nitric oxide synthase protects aortic allografts from the development of transplant arteriosclerosis.Transplantation. 2000 Mar 27;69(6):1186-92. doi: 10.1097/00007890-200003270-00025. Transplantation. 2000. PMID: 10762225
-
Role of nitric oxide in experimental obliterative bronchiolitis (chronic rejection) in the rat.J Clin Invest. 1997 Dec 15;100(12):2984-94. doi: 10.1172/JCI119852. J Clin Invest. 1997. PMID: 9399944 Free PMC article.
-
Inducible nitric oxide synthase promotes cytokine expression in cardiac allografts but is not required for efficient rejection.J Heart Lung Transplant. 1999 Sep;18(9):819-27. doi: 10.1016/s1053-2498(99)00052-2. J Heart Lung Transplant. 1999. PMID: 10528743
-
Role of inducible nitric oxide synthase in transplant arteriosclerosis.Clin Exp Pharmacol Physiol. 1999 Dec;26(12):1013-5. doi: 10.1046/j.1440-1681.1999.03183.x. Clin Exp Pharmacol Physiol. 1999. PMID: 10626073 Review.
Cited by
-
The complex role of iNOS in acutely rejecting cardiac transplants.Free Radic Biol Med. 2008 Apr 15;44(8):1536-52. doi: 10.1016/j.freeradbiomed.2008.01.020. Epub 2008 Feb 7. Free Radic Biol Med. 2008. PMID: 18291116 Free PMC article. Review.
-
Monotherapy With Anti-CD70 Antibody Causes Long-Term Mouse Cardiac Allograft Acceptance With Induction of Tolerogenic Dendritic Cells.Front Immunol. 2021 Feb 19;11:555996. doi: 10.3389/fimmu.2020.555996. eCollection 2020. Front Immunol. 2021. PMID: 33737923 Free PMC article.
-
Complement-mediated microvascular injury leads to chronic rejection.Adv Exp Med Biol. 2013;735:233-46. doi: 10.1007/978-1-4614-4118-2_16. Adv Exp Med Biol. 2013. PMID: 23402031 Free PMC article. Review.
-
Reciprocal regulation of airway rejection by the inducible gas-forming enzymes heme oxygenase and nitric oxide synthase.J Exp Med. 2005 Jul 18;202(2):283-94. doi: 10.1084/jem.20050377. J Exp Med. 2005. PMID: 16027238 Free PMC article.
-
Obligatory role for interleukin-13 in obstructive lesion development in airway allografts.Am J Pathol. 2006 Jul;169(1):47-60. doi: 10.2353/ajpath.2006.050975. Am J Pathol. 2006. PMID: 16816360 Free PMC article.
References
-
- Berry, G.J., E.M. Brunt, D. Chamberlain, R.H. Hruban, R.K. Sibley, S. Stewart, and H.D. Tazelaar. 1990. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: lung rejection study group. J. Heart Transplant. 9:593–601. - PubMed
-
- Trulock, E.P. 1997. Lung transplantation. Am. J. Respir. Crit. Care Med. 155:789–818. - PubMed
-
- Hruban, R.H., W.E. Beschorner, W.A. Baumgartner, S.C. Achuff, T.A. Traill, B.R. Marsh, P.K. Gupta, G.M. Hutchins, and B.A. Reitz. 1988. Diagnosis of lung allograft rejection by bronchial intraepithelial Leu-7 positive T lymphocytes. J. Thorac. Cardiovasc. Surg. 96:939–946. - PubMed
-
- Yousem, S.A. 1993. Lymphocytic bronchitis/bronchiolitis in lung allograft recipients. Am. J. Surg. Pathol. 17:491–496. - PubMed
-
- Yousem, S.A., G.J. Berry, P.T. Cagle, D. Chamberlain, A.N. Husain, R.H. Hruban, A. Marchevsky, N.P. Ohori, J. Ritter, S. Stewart, et al. 1996. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: lung rejection study group. J. Heart Lung Transplant. 15:1–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

