Activation-induced modification in the CD3 complex of the gammadelta T cell receptor
- PMID: 12438426
- PMCID: PMC2193986
- DOI: 10.1084/jem.20021196
Activation-induced modification in the CD3 complex of the gammadelta T cell receptor
Erratum in
- J Exp Med 2002 Dec 16;196(12):1653
Abstract
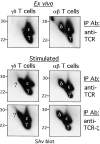
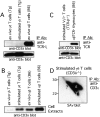
The T cell antigen receptor complexes expressed on alphabeta and gammadelta T cells differ not only in their respective clonotypic heterodimers but also in the subunit composition of their CD3 complexes. The gammadelta T cell receptors (TCRs) expressed on ex vivo gammadelta T cells lack CD3delta, whereas alphabeta TCRs contain CD3delta. While this result correlates with the phenotype of CD3delta(-/-) mice, in which gammadelta T cell development is unaffected, it is inconsistent with the results of previous studies reporting that CD3delta is a component of the gammadelta TCR. Since earlier studies examined the subunit composition of gammadelta TCRs expressed on activated and expanded peripheral gammadelta T cells or gammadelta TCR(+) intestinal intraepithelial lymphocytes, we hypothesized that activation and expansion may lead to changes in the CD3 subunit composition of the gammadelta TCR. Here, we report that activation and expansion do in fact result in the inclusion of a protein, comparable in mass and mobility to CD3delta, in the gammadelta TCR. Further analyses revealed that this protein is not CD3delta, but instead is a differentially glycosylated form of CD3gamma. These results provide further evidence for a major difference in the subunit composition of alphabeta- and gammadelta TCR complexes and raise the possibility that modification of CD3gamma may have important functional consequences in activated gammadelta T cells.
Figures



Similar articles
-
Different composition of the human and the mouse gammadelta T cell receptor explains different phenotypes of CD3gamma and CD3delta immunodeficiencies.J Exp Med. 2007 Oct 29;204(11):2537-44. doi: 10.1084/jem.20070782. Epub 2007 Oct 8. J Exp Med. 2007. PMID: 17923503 Free PMC article.
-
On the role of CD3delta chains in TCRgammadelta/CD3 complexes during assembly and membrane expression.Scand J Immunol. 2001 Jul-Aug;54(1-2):155-62. doi: 10.1046/j.1365-3083.2001.00938.x. Scand J Immunol. 2001. PMID: 11439162
-
Human CD3γ, but not CD3δ, haploinsufficiency differentially impairs γδ versus αβ surface TCR expression.BMC Immunol. 2013 Jan 21;14:3. doi: 10.1186/1471-2172-14-3. BMC Immunol. 2013. PMID: 23336327 Free PMC article.
-
αβ and γδ T cell receptors: Similar but different.J Leukoc Biol. 2020 Jun;107(6):1045-1055. doi: 10.1002/JLB.2MR1219-233R. Epub 2020 Jan 29. J Leukoc Biol. 2020. PMID: 31994778 Review.
-
An architectural perspective on signaling by the pre-, alphabeta and gammadelta T cell receptors.Immunol Rev. 2003 Feb;191:28-37. doi: 10.1034/j.1600-065x.2003.00011.x. Immunol Rev. 2003. PMID: 12614349 Review.
Cited by
-
TRAIL-R1-Targeted CAR-T Cells Exhibit Dual Antitumor Efficacy.Front Mol Biosci. 2021 Dec 20;8:756599. doi: 10.3389/fmolb.2021.756599. eCollection 2021. Front Mol Biosci. 2021. PMID: 34988114 Free PMC article.
-
Gamma Delta TCR and the WC1 Co-Receptor Interactions in Response to Leptospira Using Imaging Flow Cytometry and STORM.Front Immunol. 2021 Jul 28;12:712123. doi: 10.3389/fimmu.2021.712123. eCollection 2021. Front Immunol. 2021. PMID: 34394114 Free PMC article.
-
TCR signal strength controls thymic differentiation of discrete proinflammatory γδ T cell subsets.Nat Immunol. 2016 Jun;17(6):721-727. doi: 10.1038/ni.3424. Epub 2016 Apr 4. Nat Immunol. 2016. PMID: 27043412 Free PMC article.
-
CD3γ/δ in sea bass (Dicentrarchus labrax): Molecular characterization and expression analysis.Results Immunol. 2011 Aug 30;1(1):31-5. doi: 10.1016/j.rinim.2011.08.003. eCollection 2011. Results Immunol. 2011. PMID: 24371550 Free PMC article.
-
Different composition of the human and the mouse gammadelta T cell receptor explains different phenotypes of CD3gamma and CD3delta immunodeficiencies.J Exp Med. 2007 Oct 29;204(11):2537-44. doi: 10.1084/jem.20070782. Epub 2007 Oct 8. J Exp Med. 2007. PMID: 17923503 Free PMC article.
References
-
- Guy-Grand, D., B. Rocha, P. Mintz, M. Malassis-Seris, F. Selz, B Malissen, and P. Vassalli. 1994. Different use of T cell receptor transducing modules in two populations of gut intraepithelial lymphocytes are related to distinct pathways of T cell differentiation. J. Exp. Med. 180:673–679. - PMC - PubMed
-
- Heiken, H., R.-J. Schulz, J.V. Ravetch, E.L. Reinherz, and S. Koyasu. 1996. T lymphocyte development in the absence of Fcɛ receptor Iγ subunit: analysis of thymic-dependent and independent αβ and γδ pathways. Eur. J. Immunol. 26:1935–1943. - PubMed

