In vivo activation of cAMP signaling induces growth arrest and differentiation in acute promyelocytic leukemia
- PMID: 12438428
- PMCID: PMC2193985
- DOI: 10.1084/jem.20021129
In vivo activation of cAMP signaling induces growth arrest and differentiation in acute promyelocytic leukemia
Abstract
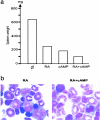
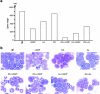
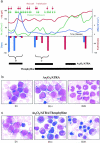
Differentiation therapy for acute myeloid leukemia uses transcriptional modulators to reprogram cancer cells. The most relevant clinical example is acute promyelocytic leukemia (APL), which responds dramatically to either retinoic acid (RA) or arsenic trioxide (As(2)O(3)). In many myeloid leukemia cell lines, cyclic adenosine monophosphate (cAMP) triggers growth arrest, cell death, or differentiation, often in synergy with RA. Nevertheless, the toxicity of cAMP derivatives and lack of suitable models has hampered trials designed to assess the in vivo relevance of theses observations. We show that, in an APL cell line, cAMP analogs blocked cell growth and unraveled As(2)O(3)-triggered differentiation. Similarly, in RA-sensitive or RA-resistant mouse models of APL, continuous infusions of 8-chloro-cyclic adenosine monophosphate (8-Cl-cAMP) triggered major growth arrest, greatly enhanced both spontaneous and RA- or As(2)O(3)-induced differentiation and accelerated the restoration of normal hematopoiesis. Theophylline, a well-tolerated phosphodiesterase inhibitor which stabilizes endogenous cAMP, also impaired APL growth and enhanced spontaneous or As(2)O(3)-triggered cell differentiation in vivo. Accordingly, in an APL patient resistant to combined RA-As(2)O(3) therapy, theophylline induced blast clearance and restored normal hematopoiesis. Taken together, these results demonstrate that in vivo activation of cAMP signaling contributes to APL clearance, independently of its RA-sensitivity, thus raising hopes that other myeloid leukemias may benefit from this therapeutic approach.
Figures


References
-
- Look, A.T. 1997. Oncogenic transcription factors in the human acute leukemias. Science. 278:1059–1064. - PubMed
-
- Minucci, S., C. Nervi, F. Lo Coco, and P.G. Pelicci. 2001. Histone deacetylases: a common molecular target for differentiation treatment of acute myeloid leukemias? Oncogene. 20:3110–3115. - PubMed
-
- Warrell, R.P., L.-Z. He, V. Richon, E. Calleja, and P.P. Pandolfi. 1998. Therapeutic targeting of transcription in acute promyelocytic leukemia by use of an inhibitor of histone deacetylase. J. Natl. Cancer Inst. 90:1621–1625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

