Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood
- PMID: 12438429
- PMCID: PMC2193991
- DOI: 10.1084/jem.20020943
Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood
Abstract
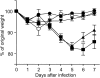
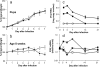
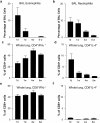
Infants experiencing severe respiratory syncytial virus (RSV) bronchiolitis have an increased frequency of wheeze and asthma in later childhood. Since most severe RSV infections occur between the 8th and 24th postnatal week, we examined whether age at first infection determines the balance of cytokine production and lung pathology during subsequent rechallenge. Primary RSV infection in newborn mice followed the same viral kinetics as in adults but was associated with reduced and delayed IFN-gamma responses. To study rechallenge, mice were infected at 1 day or 1, 4, or 8 weeks of age and reinfected at 12 weeks. Neonatal priming produced more severe weight loss and increased inflammatory cell recruitment (including T helper 2 cells and eosinophils) during reinfection, whereas delayed priming led to enhanced interferon gamma production and less severe disease during reinfection. These results show the crucial importance of age at first infection in determining the outcome of reinfection and suggest that the environment of the neonatal lung is a major determinant of cytokine production and disease patterns in later life. Thus, simply delaying RSV infection beyond infancy might reduce subsequent respiratory morbidity in later childhood.
Figures
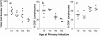

Comment in
-
Interactions between RSV infection, asthma, and atopy: unraveling the complexities.J Exp Med. 2002 Nov 18;196(10):1271-5. doi: 10.1084/jem.20021572. J Exp Med. 2002. PMID: 12438419 Free PMC article. No abstract available.
References
-
- Sarzotti, M., D.S. Robbins, and P.M. Hoffman. 1996. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 271:1726–1728. - PubMed
-
- Ridge, J.P., E.J. Fuchs, and P. Matzinger. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 271:1723–1726. - PubMed
-
- Forsthuber, T., H.C. Yip, and P.V. Lehmann. 1996. Induction of T-H1 and T-H2 immunity in neonatal mice. Science. 271:1728–1730. - PubMed
-
- Rowe, J., C. Macaubas, T. Monger, B.J. Holt, J. Harvey, J.T. Poolman, R. Loh, P.D. Sly, and P.G. Holt. 2001. Heterogeneity in diphtheria-tetanus-acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic Th1 function. J. Infect. Dis. 184:80–88. - PubMed
-
- Adkins, B. 1999. T-cell function in newborn mice and humans. Immunol. Today. 20:330–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

