IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism
- PMID: 12438449
- PMCID: PMC151808
- DOI: 10.1172/JCI15564
IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism
Abstract
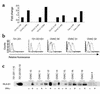
IFN-gamma regulates the immunogenicity of target cells by increasing their expression of HLA class I molecules. This facilitates the T cell receptor-mediated recognition by CD8(+) T cells but decreases target cell sensitivity to lysis by NK cells due to engagement of inhibitory NK receptors. In this study, short-term tumor cell lines from patients with advanced ovarian carcinomas were established. We demonstrate the paradoxical finding that IFN-gamma treatment of these short-term ovarian carcinoma cell lines (OVACs) resulted in resistance of tumor cells to lysis by peptide- and allospecific CD8(+) T cells. Blocking experiments revealed that this phenomenon was dependent on enhanced inhibitory signalling via CD94/NKG2A receptors expressed on the effector cells. This was associated with increased expression of HLA-E mRNA and HLA-G at the protein level in IFN-gamma-treated OVACs. Furthermore, pulsing of untreated OVACs with the leader sequence peptide of HLA-G protected these cells from lysis by CTLs, thus mimicking the inhibitory effect of IFN-gamma. This study provides evidence that CD94/NKG2A receptors play an important role in regulating T cell activity against tumors and shows that IFN-gamma modulation of target cells may shift the balance of triggering and inhibitory signals to T cells, turning off their cytolytic activity.
Figures
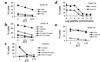


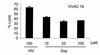
Comment in
-
IFN-gamma suspends the killing license of anti-tumor CTLs.J Clin Invest. 2002 Nov;110(10):1407-9. doi: 10.1172/JCI17209. J Clin Invest. 2002. PMID: 12438437 Free PMC article. Review. No abstract available.
References
-
- Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. - PubMed
-
- Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. - PubMed
-
- Rock KL, Goldberg AL. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–779. - PubMed
-
- Moretta A, et al. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

