Effects of membrane potential and sphingolipid structures on fusion of Semliki Forest virus
- PMID: 12438595
- PMCID: PMC136663
- DOI: 10.1128/jvi.76.24.12691-12702.2002
Effects of membrane potential and sphingolipid structures on fusion of Semliki Forest virus
Abstract
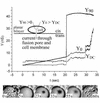
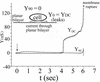
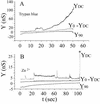
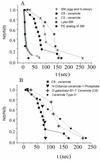
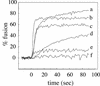
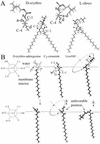
Cells expressing the E1 and E2 envelope proteins of Semliki Forest virus (SFV) were fused to voltage-clamped planar lipid bilayer membranes at low pH. Formation and evolution of fusion pores were electrically monitored by capacitance measurements, and membrane continuity was tracked by video fluorescence microscopy by including rhodamine-phosphatidylethanolamine in the bilayer. Fusion occurred without leakage for a negative potential applied to the trans side of the planar membrane. When a positive potential was applied, leakage was severe, obscuring the observation of any fusion. E1-mediated cell-cell fusion occurred without leakage for negative intracellular potentials but with substantial leakage for zero membrane potential. Thus, negative membrane potentials are generally required for nonleaky fusion. With planar bilayers as the target, the first fusion pore that formed almost always enlarged; pore flickering was a rare event. Similar to other target membranes, fusion required cholesterol and sphingolipids in the planar membrane. Sphingosine did not support fusion, but both ceramide, with even a minimal acyl chain (C(2)-ceramide), and lysosphingomyelin (lyso-SM) promoted fusion with the same kinetics. Thus, unrelated modifications to different parts of sphingosine yielded sphingolipids that supported fusion to the same degree. Fusion studies of pyrene-labeled SFV with cholesterol-containing liposomes showed that C(2)-ceramide supported fusion while lyso-SM did not, apparently due to its positive curvature effects. A model is proposed in which the hydroxyls of C-1 and C-3 as well as N of C-2 of the sphingosine backbone must orient so as to form multiple hydrogen bonds to amino acids of SFV E1 for fusion to proceed.
Figures


Similar articles
-
Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone.J Virol. 1995 May;69(5):3220-3. doi: 10.1128/JVI.69.5.3220-3223.1995. J Virol. 1995. PMID: 7707555 Free PMC article.
-
Fusion of Semliki Forest virus with cholesterol-containing liposomes at low pH: a specific requirement for sphingolipids.Mol Membr Biol. 1995 Jan-Mar;12(1):143-9. doi: 10.3109/09687689509038510. Mol Membr Biol. 1995. PMID: 7767374
-
Membrane fusion activity of Semliki Forest virus in a liposomal model system: specific inhibition by Zn2+ ions.Virology. 1997 Nov 10;238(1):14-21. doi: 10.1006/viro.1997.8799. Virology. 1997. PMID: 9375004
-
Sphingolipids activate membrane fusion of Semliki Forest virus in a stereospecific manner.Biochemistry. 1995 Aug 22;34(33):10319-24. doi: 10.1021/bi00033a001. Biochemistry. 1995. PMID: 7654685
-
Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane.EMBO J. 1994 Jun 15;13(12):2797-804. doi: 10.1002/j.1460-2075.1994.tb06573.x. EMBO J. 1994. PMID: 8026464 Free PMC article.
Cited by
-
Rapid membrane fusion of individual virus particles with supported lipid bilayers.Biophys J. 2007 Jul 15;93(2):526-38. doi: 10.1529/biophysj.106.097485. Epub 2007 Apr 20. Biophys J. 2007. PMID: 17449662 Free PMC article.
-
Induction of Cell-Cell Fusion by Ebola Virus Glycoprotein: Low pH Is Not a Trigger.PLoS Pathog. 2016 Jan 5;12(1):e1005373. doi: 10.1371/journal.ppat.1005373. eCollection 2016 Jan. PLoS Pathog. 2016. PMID: 26730950 Free PMC article.
-
Pseudorevertants of a Semliki forest virus fusion-blocking mutation reveal a critical interchain interaction in the core trimer.J Virol. 2010 Nov;84(22):11624-33. doi: 10.1128/JVI.01625-10. Epub 2010 Sep 8. J Virol. 2010. PMID: 20826687 Free PMC article.
-
Negative potentials across biological membranes promote fusion by class II and class III viral proteins.Mol Biol Cell. 2010 Jun 15;21(12):2001-12. doi: 10.1091/mbc.e09-10-0904. Epub 2010 Apr 28. Mol Biol Cell. 2010. PMID: 20427575 Free PMC article.
-
Membrane Sphingomyelin in Host Cells Is Essential for Nucleocapsid Penetration into the Cytoplasm after Hemifusion during Rubella Virus Entry.mBio. 2022 Dec 20;13(6):e0169822. doi: 10.1128/mbio.01698-22. Epub 2022 Nov 8. mBio. 2022. PMID: 36346228 Free PMC article.
References
-
- Akeson, M., J. Scharff, C. M. Sharp, and D. M. Neville, Jr. 1992. Evidence that plasma membrane electrical potential is required for vesicular stomatitis virus infection of MDCK cells: a study using fluorescence measurements through polycarbonate supports. J. Membr. Biol. 125:81-91. - PubMed
-
- Bruzik, K. S. 1988. Conformation of the polar head group of sphingomyelin and its analogues. Biochim. Biophys. Acta 939:315-326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

