Detection of the potyviral genome-linked protein VPg in virions and its phosphorylation by host kinases
- PMID: 12438596
- PMCID: PMC136665
- DOI: 10.1128/jvi.76.24.12703-12711.2002
Detection of the potyviral genome-linked protein VPg in virions and its phosphorylation by host kinases
Abstract
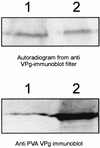
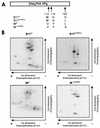
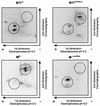
The multifunctional genome-linked protein (VPg) of Potato virus A (PVA; genus Potyvirus) was found to be phosphorylated as a part of the virus particle by a cellular kinase activity from tobacco. Immunoprecipitation, immunolabeling, and immunoelectron microscopy experiments showed that VPg is exposed at one end of the virion and it is accessible to protein-protein interactions. Substitution Ser185Leu at the C-proximal part of VPg reduces accumulation of PVA in inoculated leaves of the wild potato species Solanum commersonii and delays systemic infection, which is not observed in tobacco plants. Our data show that kinases of S. commersonii differentially recognize the VPg containing Ser or Leu at position 185, whereas both forms of VPg are similarly recognized by tobacco kinases. Taken together, our data imply that the virion-bound VPg may interact with host proteins and that phosphorylation of VPg may play a role in the VPg-mediated functions during the infection cycle of potyviruses.
Figures



Similar articles
-
Identification of the genome-linked protein in virions of Potato virus A, with comparison to other members in genus Potyvirus.Virus Res. 2001 Mar;73(2):103-12. doi: 10.1016/s0168-1702(00)00216-1. Virus Res. 2001. PMID: 11172914
-
Viral genome-linked protein (VPg) controls accumulation and phloem-loading of a potyvirus in inoculated potato leaves.Mol Plant Microbe Interact. 2002 Feb;15(2):138-49. doi: 10.1094/MPMI.2002.15.2.138. Mol Plant Microbe Interact. 2002. PMID: 11878318
-
Localization of a potyvirus and the viral genome-linked protein in wild potato leaves at an early stage of systemic infection.Mol Plant Microbe Interact. 2003 Jan;16(1):25-34. doi: 10.1094/MPMI.2003.16.1.25. Mol Plant Microbe Interact. 2003. PMID: 12580279
-
Silencing suppressor protein VPg of a potyvirus interacts with the plant silencing-related protein SGS3.Mol Plant Microbe Interact. 2014 Nov;27(11):1199-210. doi: 10.1094/MPMI-04-14-0109-R. Mol Plant Microbe Interact. 2014. PMID: 25099340
-
The genome-linked protein VPg of plant viruses-a protein with many partners.Curr Opin Virol. 2011 Nov;1(5):347-54. doi: 10.1016/j.coviro.2011.09.010. Epub 2011 Oct 14. Curr Opin Virol. 2011. PMID: 22440836 Review.
Cited by
-
Intracellular coordination of potyviral RNA functions in infection.Front Plant Sci. 2014 Mar 26;5:110. doi: 10.3389/fpls.2014.00110. eCollection 2014. Front Plant Sci. 2014. PMID: 24723931 Free PMC article. Review.
-
Dynamin-Like Proteins of Endocytosis in Plants Are Coopted by Potyviruses To Enhance Virus Infection.J Virol. 2018 Nov 12;92(23):e01320-18. doi: 10.1128/JVI.01320-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30258010 Free PMC article.
-
A novel role of the potyviral helper component proteinase contributes to enhance the yield of viral particles.J Virol. 2014 Sep 1;88(17):9808-18. doi: 10.1128/JVI.01010-14. Epub 2014 Jun 18. J Virol. 2014. PMID: 24942578 Free PMC article.
-
Inspirations on Virus Replication and Cell-to-Cell Movement from Studies Examining the Cytopathology Induced by Lettuce infectious yellows virus in Plant Cells.Front Plant Sci. 2017 Sep 27;8:1672. doi: 10.3389/fpls.2017.01672. eCollection 2017. Front Plant Sci. 2017. PMID: 29021801 Free PMC article. Review.
-
A cysteine-rich plant protein potentiates Potyvirus movement through an interaction with the virus genome-linked protein VPg.J Virol. 2004 Mar;78(5):2301-9. doi: 10.1128/jvi.78.5.2301-2309.2004. J Virol. 2004. PMID: 14963126 Free PMC article.
References
-
- Andrejeva, J., Ü. Puurand, A. Merits, F. Rabenstein, L. Järvekülg, and J. P. T. Valkonen. 1999. Potyvirus helper component-proteinase and coat protein (CP) have coordinated functions in virus-host interactions and the same CP motif affects virus transmission and accumulation. J. Gen. Virol. 80:1133-1139. - PubMed
-
- Atabekov, J. G., N. P. Radionova, O. V. Karpova, S. V. Kozlowsky, V. K. Novikov, and M. V. Arkhipenko. 2001. Translational activation of encapsidated potato virus X RNA by coat protein phosphorylation. Virology 286:466-474. - PubMed
-
- Blume-Jensen, P., C. Wernsted, C.-H. Heldin, and L. Rönnstrand. 1995. Identification of the major phosphorylation sites for protein kinase C in kit/stem cell factor receptor in vitro and in intact cells. J. Biol. Chem. 270:14192-14200. - PubMed
-
- Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-148. - PubMed
-
- Browning, I. A., R. Burns, E. L. George, and M. Darling. 1995. Development and evaluation of ELISA assays incorporating monoclonal antibodies for the detection of potato A potyvirus. EPPO Bull. 25:259-268.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

