Tissue-specific transcriptional targeting of a replication-competent retroviral vector
- PMID: 12438603
- PMCID: PMC136666
- DOI: 10.1128/jvi.76.24.12783-12791.2002
Tissue-specific transcriptional targeting of a replication-competent retroviral vector
Abstract
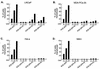
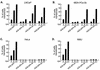
The inability of replication-defective viral vectors to efficiently transduce tumor cells in vivo has prevented the successful application of such vectors in gene therapy of cancer. To address the need for more efficient gene delivery systems, we have developed replication-competent retroviral (RCR) vectors based on murine leukemia virus (MLV). We have previously shown that such vectors are capable of transducing solid tumors in vivo with very high efficiency. While the natural requirement of MLV infection for cell division imparts a certain degree of specificity for tumor cells, additional means for confining RCR vector replication to tumor cells are desirable. Here, we investigated the parameters critical for successful tissue-specific transcriptional control of RCR vector replication by replacing various lengths of the MLV enhancer/promoter with sequences derived either from the highly prostate-specific probasin (PB) promoter or from a more potent synthetic variant of the PB promoter. We assessed the transcriptional specificity of the resulting hybrid long terminal repeats (LTRs) and the cell type specificity and efficiency of replication of vectors containing these LTRs. Incorporation of PB promoter sequences effectively restricted transcription from the LTR to prostate-derived cells and imparted prostate-specific RCR vector replication but required the stronger synthetic promoter and retention of native MLV sequences in the vicinity of the TATA box for optimal replicative efficiency and specificity. Our results have thus identified promoter strength and positioning within the LTR as important determinants for achieving both high transduction efficiency and strict cell type specificity in transcriptionally targeted RCR vectors.
Figures

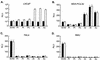

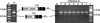
Similar articles
-
Tissue- and tumor-specific targeting of murine leukemia virus-based replication-competent retroviral vectors.J Virol. 2006 Jul;80(14):7070-8. doi: 10.1128/JVI.00020-06. J Virol. 2006. PMID: 16809312 Free PMC article.
-
Construction of replication-competent oncolytic retroviral vectors expressing R peptide-truncated 10A1 envelope glycoprotein.J Virol Methods. 2019 Jun;268:32-36. doi: 10.1016/j.jviromet.2019.03.008. Epub 2019 Mar 18. J Virol Methods. 2019. PMID: 30898575
-
Antibody-mediated targeting of replication-competent retroviral vectors.Hum Gene Ther. 2003 May 20;14(8):789-802. doi: 10.1089/104303403765255174. Hum Gene Ther. 2003. PMID: 12804141
-
Beyond oncolytic virotherapy: replication-competent retrovirus vectors for selective and stable transduction of tumors.Curr Gene Ther. 2005 Dec;5(6):655-67. doi: 10.2174/156652305774964659. Curr Gene Ther. 2005. PMID: 16457654 Review.
-
Replication-competent retrovirus vectors for cancer gene therapy.Front Biosci. 2008 Jan 1;13:3083-95. doi: 10.2741/2910. Front Biosci. 2008. PMID: 17981778 Review.
Cited by
-
Tissue- and tumor-specific targeting of murine leukemia virus-based replication-competent retroviral vectors.J Virol. 2006 Jul;80(14):7070-8. doi: 10.1128/JVI.00020-06. J Virol. 2006. PMID: 16809312 Free PMC article.
-
Influence of vector design and host cell on the mechanism of recombination and emergence of mutant subpopulations of replicating retroviral vectors.BMC Mol Biol. 2009 Feb 9;10:8. doi: 10.1186/1471-2199-10-8. BMC Mol Biol. 2009. PMID: 19203366 Free PMC article.
-
Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression.Mol Ther. 2012 Sep;20(9):1689-1698. doi: 10.1038/mt.2012.83. Epub 2012 May 1. Mol Ther. 2012. PMID: 22547150 Free PMC article.
-
MiRNA inhibition in tissue engineering and regenerative medicine.Adv Drug Deliv Rev. 2015 Jul 1;88:123-37. doi: 10.1016/j.addr.2014.12.006. Epub 2014 Dec 29. Adv Drug Deliv Rev. 2015. PMID: 25553957 Free PMC article. Review.
-
Effects of viral strain, transgene position, and target cell type on replication kinetics, genomic stability, and transgene expression of replication-competent murine leukemia virus-based vectors.J Virol. 2007 Jul;81(13):6973-83. doi: 10.1128/JVI.02470-06. Epub 2007 Apr 18. J Virol. 2007. PMID: 17442710 Free PMC article.
References
-
- Andriani, F., B. Nan, J. Yu, X. Li, N. L. Weigel, M. J. McPhaul, S. Kasper, S. Kagawa, B. Fang, R. J. Matusik, L. Denner, and M. Marcelli. 2001. Use of the probasin promoter ARR2PB to express Bax in androgen receptor-positive prostate cancer cells. J. Natl. Cancer Inst. 93:1314-1324. - PubMed
-
- Benz, E. W., Jr., R. M. Wydro, B. Nadal-Ginard, and D. Dina. 1980. Moloney murine sarcoma proviral DNA is a transcriptional unit. Nature 288:665-669. - PubMed
-
- Cavazzana-Calvo, M., S. Hacein-Bey, G. de Saint Basile, F. Gross, E. Yvon, P. Nusbaum, F. Selz, C. Hue, S. Certain, J. L. Casanova, P. Bousso, F. L. Deist, and A. Fischer. 2000. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288:669-672. - PubMed
-
- Cohen, L. A. 1982. Isolation and characterization of a serially cultivated, neoplastic, epithelial cell line from the N-nitrosomethylurea induced rat mammary adenocarcinoma. In Vitro 18:565-575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

