Requirement for vacuolar H+ -ATPase activity and Ca2+ gradient during entry of rotavirus into MA104 cells
- PMID: 12438636
- PMCID: PMC136671
- DOI: 10.1128/jvi.76.24.13083-13087.2002
Requirement for vacuolar H+ -ATPase activity and Ca2+ gradient during entry of rotavirus into MA104 cells
Abstract
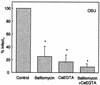
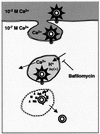
The mechanism by which rotavirus and other nonenveloped viruses enter the cell is still not clear. We have proposed an endocytosis model where the critical step for virus uncoating and membrane permeabilization is the decrease in Ca(2+) concentration in the endosome. In this paper, we monitored rotavirus entry by measuring alpha-sarcin-rotavirus coentry and infectivity in MA104 cells. The participation of endocytosis, acidification, and endosomal Ca(2+) concentration on virus entry was studied by inhibiting the endosomal H(+)-ATPase with bafilomycin A1 and/or increasing the extracellular calcium reservoir by addition of 10 mM CaEGTA. Rotavirus-alpha-sarcin coentry was inhibited by bafilomycin A1 and by addition of 10 mM CaEGTA. These effects were additive. These substances induced a significant inhibition of infectivity without affecting virus binding and postentry steps. These results are compatible with the interpretation that bafilomycin A1 and CaEGTA block rotavirus penetration from the endosome into the cytoplasm and support our hypothesis of a Ca(2+)-dependent endocytosis model.
Figures


References
-
- Bass, D. M., M. Baylor, C. Chen, and U. Upadhyayula. 1995. Dansylcadaverine and cytochalasin d enhance rotavirus infection of murine l cells. Virology 212:429-437. - PubMed
-
- Charpilienne, A., M. J. Abad, F. Michelangeli, F. Alvarado, M. Vasseur, J. Cohen, and M. C. Ruiz. 1997. Solubilized and cleaved VP7, the outer glycoprotein of rotavirus, induces permeabilization of cell membrane vesicles. J. Gen. Virol. 78:1367-1371. - PubMed
-
- Ciarlet, M., M. Hidalgo, M. Gorziglia, and F. Liprandi. 1994. Characterization of neutralization epitopes on the VP7 surface protein of serotype G11 porcine rotaviruses. J. Gen. Virol. 75:1867-1873. - PubMed
-
- Ciarlet, M., J. E. Ludert, M. Iturriza-Gomara, F. Liprandi, J. J. Gray, U. Desselberger, and M. K. Estes. 2002. Initial interaction of rotavirus strains with N-acetylneuraminic (sialic) acid residues on the cell surface correlates with VP4 genotype, not species of origin. J. Virol. 76:4087-4095. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

