Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI
- PMID: 12438696
- PMCID: PMC137732
- DOI: 10.1073/pnas.222421399
Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI
Abstract
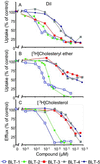
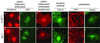
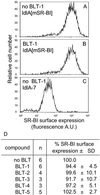
The high-density lipoprotein (HDL) receptor, scavenger receptor, class B, type I (SR-BI), mediates both the selective uptake of lipids, mainly cholesterol esters, from HDL to cells and the efflux of cholesterol from cells to lipoproteins. The mechanism underlying these lipid transfers is distinct from classic receptor-mediated endocytosis, but it remains poorly understood. To investigate SR-BI's mechanism of action and in vivo function, we developed a high-throughput screen to identify small molecule inhibitors of SR-BI-mediated lipid transfer in intact cells. We identified five compounds that in the low nanomolar to micromolar range block lipid transport (BLTs), both selective uptake and efflux. The effects of these compounds were highly specific to the SR-BI pathway, because they didn't interfere with receptor-mediated endocytosis or with other forms of intracellular vesicular traffic. Surprisingly, all five BLTs enhanced, rather than inhibited, HDL binding by increasing SR-BI's binding affinity for HDL (decreased dissociation rates). Thus, the BLTs provide strong evidence for a mechanistic coupling between HDL binding and lipid transport and may serve as a starting point for the development of pharmacologically useful modifiers of SR-BI activity and, thus, HDL metabolism.
Figures


References
-
- Acton S., Rigotti, A., Landschulz, K. T., Xu, S., Hobbs, H. H. & Krieger, M. (1996) Science 271 518-520. - PubMed
-
- Krieger M. (1999) Annu. Rev. Biochem. 68 523-558. - PubMed
-
- Kozarsky K. F., Donahee, M. H., Glick, J. M., Krieger, M. & Rader, D. J. (2000) Arterioscler. Thromb. Vasc. Biol. 20 721-727. - PubMed
-
- Arai T., Wang, N., Bezouevski, M., Welch, C. & Tall, A. R. (1999) J. Biol. Chem. 274 2366-2371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

