Partly folded states of members of the lysozyme/lactalbumin superfamily: a comparative study by circular dichroism spectroscopy and limited proteolysis
- PMID: 12441391
- PMCID: PMC2373748
- DOI: 10.1110/ps.0205802
Partly folded states of members of the lysozyme/lactalbumin superfamily: a comparative study by circular dichroism spectroscopy and limited proteolysis
Abstract
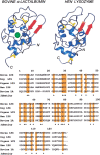
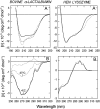
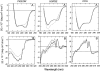
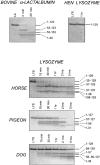
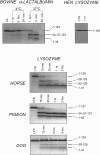
The partly folded states of protein members of the lysozyme (LYS)/alpha-lactalbumin (LA) superfamily have been analyzed by circular dichroism (CD) measurements and limited proteolysis experiments. Hen, horse, dog, and pigeon LYSs and bovine LA were used in the present study. These are related proteins of 123- to 129-amino-acid residues with similar three-dimensional structures but low similarity in amino acid sequences. Moreover, notable differences among them reside in their calcium-binding properties and capability to adopt partly folded states or molten globules in acid solution (A-state) or on depletion of calcium at neutral pH (apo-state). Far- and near-UV CD measurements revealed that although the structures of hen and dog LYS are rather stable in acid at pH 2.0 or at neutral pH in the absence of calcium, conformational transitions to various extents occur with all other LYS/LA proteins herewith investigated. The most significant perturbation of tertiary structure in acid was observed with bovine LA and LYS from horse milk and pigeon egg-white. Pepsin and proteinase K were used as proteolytic probes, because these proteases show broad substrate specificity, and therefore, their sites of proteolysis are dictated not by the specific amino acid sequence of the protein substrate but by its overall structure and dynamics. Although hen LYS at pH 2.0 was fully resistant to proteolysis by pepsin, the other members of the LYS/LA superfamily were cleaved at different rates at few sites of the polypeptide chain and thus producing rather large protein fragments. The apo-form of bovine LA, horse LYS, and pigeon LYS were attacked by proteinase K at pH 8.3, whereas dog and hen LYSs were resistant to proteolysis when reacted under identical experimental conditions. Briefly, it has been found that the proteolysis data correlate well with the extent of conformational transitions inferred from CD spectra and with existing structural informations regarding the proteins herewith investigated, mainly derived from NMR and hydrogen exchange measurements. The sites of initial proteolytic cleavages in the LYS variants occur at the level of the beta-subdomain (approximately chain region 34-57), in analogy to those observed with bovine LA. Proteolysis data are in agreement with the current view that the molten globule of the LYS/LA proteins is characterized by a structured alpha-domain and a largely disrupted beta-subdomain. Our results underscore the utility of the limited proteolysis approach for analyzing structure and dynamics of proteins, even if adopting an ensemble of dynamic states as in the molten globule.
Figures
Similar articles
-
Limited proteolysis of bovine alpha-lactalbumin: isolation and characterization of protein domains.Protein Sci. 1999 Nov;8(11):2290-303. doi: 10.1110/ps.8.11.2290. Protein Sci. 1999. PMID: 10595532 Free PMC article.
-
Molten globule of bovine alpha-lactalbumin at neutral pH induced by heat, trifluoroethanol, and oleic acid: a comparative analysis by circular dichroism spectroscopy and limited proteolysis.Proteins. 2002 Nov 15;49(3):385-97. doi: 10.1002/prot.10234. Proteins. 2002. PMID: 12360528
-
Stepwise proteolytic removal of the beta subdomain in alpha-lactalbumin. The protein remains folded and can form the molten globule in acid solution.Eur J Biochem. 2001 Aug;268(15):4324-33. doi: 10.1046/j.1432-1327.2001.02352.x. Eur J Biochem. 2001. PMID: 11488928
-
Conformational comparison between alpha-lactalbumin and lysozyme.Adv Biophys. 1994;30:37-84. doi: 10.1016/0065-227x(94)90010-8. Adv Biophys. 1994. PMID: 7709804 Review.
-
Probing protein structure by limited proteolysis.Acta Biochim Pol. 2004;51(2):299-321. Acta Biochim Pol. 2004. PMID: 15218531 Review.
Cited by
-
Modulation of physiological and pathological activities of lysozyme by biological membranes.Cell Mol Biol Lett. 2012 Sep;17(3):349-75. doi: 10.2478/s11658-012-0015-6. Epub 2012 Apr 28. Cell Mol Biol Lett. 2012. PMID: 22544762 Free PMC article.
-
The effects of molecular crowding on the amyloid fibril formation of alpha-lactalbumin and the chaperone action of alpha-casein.Protein J. 2010 May;29(4):257-64. doi: 10.1007/s10930-010-9247-3. Protein J. 2010. PMID: 20496103
-
Iron-sulfur cluster biosynthesis: characterization of a molten globule domain in human NFU.Biochemistry. 2009 Aug 11;48(31):7512-8. doi: 10.1021/bi9002524. Biochemistry. 2009. PMID: 19722697 Free PMC article.
-
A simple and rapid pipeline for identification of receptor-binding sites on the surface proteins of pathogens.Sci Rep. 2020 Jan 24;10(1):1163. doi: 10.1038/s41598-020-58305-y. Sci Rep. 2020. PMID: 31980725 Free PMC article.
-
Förster resonance energy transfer evidence for lysozyme oligomerization in lipid environment.J Phys Chem B. 2010 Dec 23;114(50):16773-82. doi: 10.1021/jp108976e. Epub 2010 Dec 2. J Phys Chem B. 2010. PMID: 21126034 Free PMC article.
References
-
- Arai, M. and Kuwajima, K. 2000. Role of the molten globule state in protein folding. Adv. Protein Chem. 53 209–282. - PubMed
-
- Blanch, E.W., Morozova-Roche, L.A., Hecht, L., Noppe, W., and Barron, L.D. 2000. Raman optical activity characterization of native and molten globule state of equine lysozyme: Comparison with hen lysozyme and bovine α-lactalbumin. Biopolymers 57 235–248. - PubMed
-
- Canfield, R.E. 1963. The amino acid sequence of egg-white lysozyme. J. Biol. Chem. 238 2698–2707. - PubMed
-
- Chamberlain, A.K. and Marqusee, S. 2000. Comparison of equilibrium and kinetic approaches for determining protein folding mechanisms. Adv. Protein Chem. 53 283–328. - PubMed
-
- Chaudhuri, T.K., Horij, K., Yoda, T., Arai, M., Nagata, S., Terada, T.P., Uchiyama, H., Ikura, T., Tsumoto, K., Kataoka, K., Mutsushima, M., Kuwajima, K., and Kumagai, I. 1999. Effect of the extra N-terminal methionine residue on the stability and folding of recombinant α-lactalbumin expressed in Escherichia coli. J. Mol. Biol. 285 1179–1194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

