Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells
- PMID: 12441403
- PMCID: PMC138572
- DOI: 10.1073/pnas.252618999
Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells
Abstract
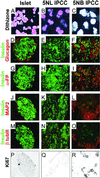
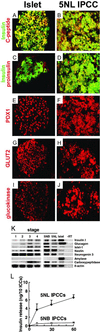
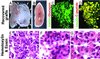
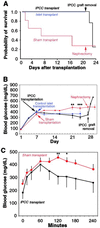
The use of embryonic stem cells for cell-replacement therapy in diseases like diabetes mellitus requires methods to control the development of multipotent cells. We report that treatment of mouse embryonic stem cells with inhibitors of phosphoinositide 3-kinase, an essential intracellular signaling regulator, produced cells that resembled pancreatic beta cells in several ways. These cells aggregated in structures similar, but not identical, to pancreatic islets of Langerhans, produced insulin at levels far greater than previously reported, and displayed glucose-dependent insulin release in vitro. Transplantation of these cell aggregates increased circulating insulin levels, reduced weight loss, improved glycemic control, and completely rescued survival in mice with diabetes mellitus. Graft removal resulted in rapid relapse and death. Graft analysis revealed that transplanted insulin-producing cells remained differentiated, enlarged, and did not form detectable tumors. These results provide evidence that embryonic stem cells can serve as the source of insulin-producing replacement tissue in an experimental model of diabetes mellitus. Strategies for producing cells that can replace islet functions described here can be adapted for similar uses with human cells.
Figures
Similar articles
-
Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo.Sci Rep. 2016 Oct 12;6:35145. doi: 10.1038/srep35145. Sci Rep. 2016. PMID: 27731367 Free PMC article.
-
Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets.Science. 2001 May 18;292(5520):1389-94. doi: 10.1126/science.1058866. Epub 2001 Apr 26. Science. 2001. PMID: 11326082
-
Differentiation of human embryonic stem cells into insulin-producing clusters.Stem Cells. 2004;22(3):265-74. doi: 10.1634/stemcells.22-3-265. Stem Cells. 2004. PMID: 15153604
-
Glucose-responsive insulin-producing cells from stem cells.Diabetes Metab Res Rev. 2002 Nov-Dec;18(6):442-50. doi: 10.1002/dmrr.330. Diabetes Metab Res Rev. 2002. PMID: 12469358 Review.
-
Generation of insulin-producing cells from stem cells.Novartis Found Symp. 2005;265:158-67; discussion 167-73, 204-11. Novartis Found Symp. 2005. PMID: 16050256 Review.
Cited by
-
Diabetes treatment: A rapid review of the current and future scope of stem cell research.Saudi Pharm J. 2015 Sep;23(4):333-40. doi: 10.1016/j.jsps.2013.12.012. Epub 2013 Dec 24. Saudi Pharm J. 2015. PMID: 27134533 Free PMC article. Review.
-
Genetically engineered islets and alternative sources of insulin-producing cells for treating autoimmune diabetes: quo vadis?Int J Endocrinol. 2012;2012:296485. doi: 10.1155/2012/296485. Epub 2012 May 29. Int J Endocrinol. 2012. PMID: 22690214 Free PMC article.
-
Insulin expressing cells from differentiated embryonic stem cells are not beta cells.Diabetologia. 2004 Mar;47(3):499-508. doi: 10.1007/s00125-004-1349-z. Epub 2004 Feb 14. Diabetologia. 2004. PMID: 14968299
-
The journey of islet cell transplantation and future development.Islets. 2018 Mar 4;10(2):80-94. doi: 10.1080/19382014.2018.1428511. Epub 2018 Feb 5. Islets. 2018. PMID: 29394145 Free PMC article. Review.
-
Differentiation of mouse embryonic stem cells into endoderm without embryoid body formation.PLoS One. 2010 Nov 30;5(11):e14146. doi: 10.1371/journal.pone.0014146. PLoS One. 2010. PMID: 21152387 Free PMC article.
References
-
- Shapiro A. M., Lakey, J. R., Ryan, E. A., Korbutt, G. S., Toth, E., Warnock, G. L., Kneteman, N. M. & Rajotte, R. V. (2000) N. Engl. J. Med. 343, 230-238. - PubMed
-
- Lumelsky N., Blondel, O., Laeng, P., Velasco, I., Ravin, R. & McKay, R. (2001) Science 292, 1389-1394. - PubMed
-
- Shiroi A., Yoshikawa, M., Yokota, H., Fukui, H., Ishizaka, S., Tatsumi, K. & Takahashi, Y. (2002) Stem Cells 20, 284-292. - PubMed
-
- Soria B., Roche, E., Berna, G., Leon-Quinto, T., Reig, J. A. & Martin, F. (2000) Diabetes 49, 157-162. - PubMed
-
- Kim S. K. & Hebrok, M. (2001) Genes Dev. 15, 111-127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

