Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion
- PMID: 12444248
- PMCID: PMC138595
- DOI: 10.1073/pnas.252582399
Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion
Abstract
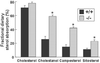
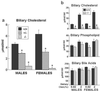
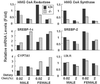
Cholesterol and other sterols exit the body primarily by secretion into bile. In patients with sitosterolemia, mutations in either of two ATP-binding cassette (ABC) half-transporters, ABCG5 or ABCG8, lead to reduced secretion of sterols into bile, implicating these transporters in this process. To elucidate the roles of ABCG5 and ABCG8 in the trafficking of sterols, we disrupted Abcg5 and Abcg8 in mice (G5G8(-/-)). The G5G8(-/-) mice had a 2- to 3-fold increase in the fractional absorption of dietary plant sterols, which was associated with an approximately 30-fold increase in plasma sitosterol. Biliary cholesterol concentrations were extremely low in the G5G8(-/-) mice when compared with wild-type animals (mean = 0.4 vs. 5.5 micromol ml) and increased only modestly with cholesterol feeding. Plasma and liver cholesterol levels were reduced by 50% in the chow-fed G5G8(-/-) mice and increased 2.4- and 18-fold, respectively, after cholesterol feeding. These data indicate that ABCG5 and ABCG8 are required for efficient secretion of cholesterol into bile and that disruption of these genes increases dramatically the responsiveness of plasma and hepatic cholesterol levels to changes in dietary cholesterol content.
Figures



References
-
- Bjorkhem I., Boberg, K. & Leitersdorf, E. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C., Beaudet, A., Sly, W. & Valle, D. (McGraw–Hill, New York), Vol. II, pp. 2961–2988.
-
- Miettinen T. A. (1980) Eur. J. Clin. Invest. 10, 27-35. - PubMed
-
- Salen G., Shore, V., Tint, G. S., Forte, T., Shefer, S., Horak, I., Horak, E., Dayal, B., Nguyen, L., Batta, A. K., et al. (1989) J. Lipid Res. 30, 1319-1330. - PubMed
-
- Morganroth J., Levy, R. I., McMahon, A. E. & Gotto, A. M., Jr. (1974) J. Pediatr. (Berlin) 85, 639-643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

