Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1
- PMID: 12444253
- PMCID: PMC138605
- DOI: 10.1073/pnas.252339399
Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1
Abstract
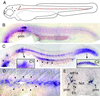
Cell migration plays an essential role in many morphogenetic processes, and its deregulation has many dramatic consequences. Yet how migration is controlled during normal development is still a largely unresolved question. We examined this process in the case of the posterior lateral line (PLL), a mechanosensory system present in fish and amphibians. In zebrafish, the embryonic PLL comprises seven to eight sense organs (neuromasts) aligned from head to tail along the flank of the animal and is formed by a primordium that originates from a cephalic placode. This primordium migrates along a stereotyped pathway toward the tip of the tail and deposits in its wake discrete groups of cells, each of which will become a neuromast. We show that a trail of SDF1-like chemokine is present along the pathway of the primordium and that a CXCR4-like chemokine receptor is expressed by the migrating cells. The inactivation of either the ligand or its receptor blocks migration, whereas in mutants in which the normal SDF1 trail is absent, the primordium path is redirected to the next, more ventral sdf1 expression domain. In all cases, the sensory axons remain associated to the primordium, indicating that the extension of the neurites to form the PLL nerve depends on the movement of the primordium. We conclude that both the formation and the innervation of this system depend on the SDF1-CXCR4 system, which has also been implicated in several migration events in humans, including metastasis formation and lymphocyte homing.
Figures

References
-
- Dijkgraaf S. (1989) in The Mechanosensory Lateral Line: Neurobiology and Evolution, eds. Coombs, S., Görner, P. & Münz, H. (Springer, New York), pp. 7–14.
-
- Metcalfe W. K. (1989) J. Comp. Neurol. 238, 218-224. - PubMed
-
- Ledent V. (2002) Development (Cambridge, U.K.) 129, 597-604. - PubMed
-
- Sapède D., Gompel, N., Dambly-Chaudière, C. & Ghysen, A. (2002) Development (Cambridge, U.K.) 129, 605-615. - PubMed
-
- Gompel N., Cubedo, N., Thisse, C., Thisse, B., Dambly-Chaudière, C. & Ghysen, A. (2001) Mech. Dev. 105, 69-77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

