Development and validation of limited sampling strategies for prediction of the systemic exposure to the novel anticancer agent E7070 (N-(3-chloro-7-indolyl)-1,4-benzenedisulphonamide)
- PMID: 12445024
- PMCID: PMC1874478
- DOI: 10.1046/j.1365-2125.2002.01684.x
Development and validation of limited sampling strategies for prediction of the systemic exposure to the novel anticancer agent E7070 (N-(3-chloro-7-indolyl)-1,4-benzenedisulphonamide)
Abstract
Aims: E7070 is a novel, sulphonamide anticancer agent currently under clinical development for the treatment of solid tumours. The aim of this study was to develop and validate limited sampling strategies for the prediction of E7070 exposure in two different treatment schedules for phase II studies using the Bayesian estimation approach.
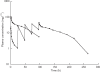
Methods: Data from two phase I dose finding studies were used in which E7070 was administered either as a single 1 h infusion or as a daily 1 h infusion for 5 days. Plasma concentration-time data from 75 patients were randomly divided into an index data set, used for the development of the strategies, and a validation data set. Population pharmacokinetic parameters were derived on the basis of the index data set. The D-optimality algorithm was used for the selection of optimal time points for both treatment schedules. The developed strategies were compared by assessment of their predictive performance of exposure, expressed as AUC (area under the plasma concentration vs time curve), in the validation data set.
Results: The developed population pharmacokinetic model comprised three compartments, with saturable distribution to one peripheral compartment and both linear and saturable elimination from the central compartment. For the 1 h infusion, a four sample strategy was selected which resulted in unbiased and accurate predictions of AUC (bias 0.74%, precision 13%). A five sample strategy was generated for the daily times five schedule yielding unbiased (bias 3.2%) and precise (12% precision) predictions of AUC.
Conclusions: Optimal sampling strategies were developed and validated for estimation of E7070 exposure in two different treatment schedules. Both schedules enabled accurate and unbiased predictions of AUC.
Figures
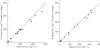
Similar articles
-
Population pharmacokinetics of the novel anticancer agent E7070 during four phase I studies: model building and validation.J Clin Oncol. 2002 Oct 1;20(19):4065-73. doi: 10.1200/JCO.2002.01.005. J Clin Oncol. 2002. PMID: 12351604
-
A comparison of limited sampling strategies for prediction of Ecteinascidin 743 clearance when administered as a 24-h infusion.Cancer Chemother Pharmacol. 2001 Dec;48(6):459-66. doi: 10.1007/s002800100368. Cancer Chemother Pharmacol. 2001. PMID: 11800026
-
Phase I and pharmacokinetic study of E7070, a chloroindolyl-sulfonamide anticancer agent, administered on a weekly schedule to patients with solid tumors.Clin Cancer Res. 2003 Nov 1;9(14):5195-204. Clin Cancer Res. 2003. PMID: 14613999 Clinical Trial.
-
Clinical pharmacokinetics and dose optimisation of carboplatin.Clin Pharmacokinet. 1997 Sep;33(3):161-83. doi: 10.2165/00003088-199733030-00002. Clin Pharmacokinet. 1997. PMID: 9314610 Review.
-
Population Pharmacokinetic Modelling and Bayesian Estimation of Tacrolimus Exposure: Is this Clinically Useful for Dosage Prediction Yet?Clin Pharmacokinet. 2016 Nov;55(11):1295-1335. doi: 10.1007/s40262-016-0396-1. Clin Pharmacokinet. 2016. PMID: 27138787 Review.
Cited by
-
Indisulam Reduces Viability and Regulates Apoptotic Gene Expression in Pediatric High-Grade Glioma Cells.Biomedicines. 2022 Dec 27;11(1):68. doi: 10.3390/biomedicines11010068. Biomedicines. 2022. PMID: 36672576 Free PMC article.
-
Application of population pharmacokinetic modeling in early clinical development of the anticancer agent E7820.Invest New Drugs. 2009 Apr;27(2):140-52. doi: 10.1007/s10637-008-9164-x. Epub 2008 Aug 20. Invest New Drugs. 2009. PMID: 18712503 Clinical Trial.
-
Omeprazole limited sampling strategies to predict area under the concentration-time curve ratios: implications for cytochrome P450 2C19 and 3A phenotyping.Eur J Clin Pharmacol. 2012 Apr;68(4):407-13. doi: 10.1007/s00228-011-1136-y. Epub 2011 Oct 19. Eur J Clin Pharmacol. 2012. PMID: 22009190 Clinical Trial.
-
Population pharmacokinetics and pharmacodynamics for treatment optimization in clinical oncology.Clin Pharmacokinet. 2008;47(8):487-513. doi: 10.2165/00003088-200847080-00001. Clin Pharmacokinet. 2008. PMID: 18611060 Review.
References
-
- Owa T, Okachi T, Yoshimatsu K, et al. A focused compound library of novel N-(7-indolyl) benzenesulfonamides for the discovery of potent cell cycle inhibitors. Bioorg Med Chem Lett. 2000;10:1223–1226. - PubMed
-
- Owa T, Yoshino H, Okauchi T, et al. Discovery of novel anti-tumor sulfonamides targeting G1 phase of the cell cycle. J Med Chem. 1999;42:3789–3799. - PubMed
-
- Fukuoka K, Usuda J, Fukumoto H, et al. Mechanism of action of a novel sulfonamide antitumor agent, E7070. Proc Am Assoc Cancer Res. 2000;41:59.
-
- Raymond E, ten Bokkel Huinink WW, Taied J. Phase I and pharmacokinetic study of E7070, a new chloro-indolysulfonamide, given as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Proc Am Assoc Cancer Res. 2000;41:611.
-
- Punt CJA, Fumoleau P, van de Walle B, Faber MN, Ravic M, Campone M. Phase I and pharmacokinetic study of E7070, a novel sulfonamide, given at a daily times five schedule in patients with advanced solid tumors. A study by the EORTC-Early Clinical Studies Group (ECSG) Ann Oncol. 2001;12:1289–1293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

