Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha
- PMID: 12446770
- PMCID: PMC139892
- DOI: 10.1128/MCB.22.24.8506-8513.2002
Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha
Abstract
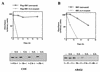
The molecular chaperone HSP90 regulates stability and function of multiple protein kinases. The HSP90-binding drug geldanamycin interferes with this activity and promotes proteasome-dependent degradation of most HSP90 client proteins. Geldanamycin also binds to GRP94, the HSP90 paralog located in the endoplasmic reticulum (ER). Because two of three ER stress sensors are transmembrane kinases, namely IRE1alpha and PERK, we investigated whether HSP90 is necessary for the stability and function of these proteins. We found that HSP90 associates with the cytoplasmic domains of both kinases. Both geldanamycin and the HSP90-specific inhibitor, 514, led to the dissociation of HSP90 from the kinases and a concomitant turnover of newly synthesized and existing pools of these proteins, demonstrating that the continued association of HSP90 with the kinases was required to maintain their stability. Further, the previously reported ability of geldanamycin to stimulate ER stress-dependent transcription apparently depends on its interaction with GRP94, not HSP90, since geldanamycin but not 514 led to up-regulation of BiP. However, this effect is eventually superseded by HSP90-dependent destabilization of unfolded protein response signaling. These data establish a role for HSP90 in the cellular transcriptional response to ER stress and demonstrate that chaperone systems on both sides of the ER membrane serve to integrate this signal transduction cascade.
Figures





References
-
- Argon, Y., and B. B. Simen. 1999. GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol. 10:495-505. - PubMed
-
- Bertolotti, A., Y. Zhang, L. M. Hendershot, H. P. Harding, and D. Ron. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2:326-332. - PubMed
-
- Calfon, M., H. Zeng, F. Urano, J. H. Till, S. R. Hubbard, H. P. Harding, S. G. Clark, and D. Ron. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92-96. - PubMed
-
- Carrell, R. W., and D. A. Lomas. 1997. Conformational disease. Lancet 350:134-138. - PubMed
-
- Chapman, R., C. Sidrauski, and P. Walter. 1998. Intracellular signaling from the endoplasmic reticulum to the nucleus. Annu. Rev. Cell Dev. Biol. 14:459-485. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
