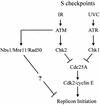
An ATR- and Chk1-dependent S checkpoint inhibits replicon initiation following UVC-induced DNA damage
- PMID: 12446774
- PMCID: PMC139882
- DOI: 10.1128/MCB.22.24.8552-8561.2002
An ATR- and Chk1-dependent S checkpoint inhibits replicon initiation following UVC-induced DNA damage
Abstract
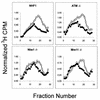
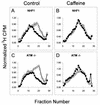
Inhibition of replicon initiation is a stereotypic DNA damage response mediated through S checkpoint mechanisms not yet fully understood. Studies were undertaken to elucidate the function of checkpoint proteins in the inhibition of replicon initiation following irradiation with 254 nm UV light (UVC) of diploid human fibroblasts immortalized by the ectopic expression of telomerase. Velocity sedimentation analysis of nascent DNA molecules revealed a 50% inhibition of replicon initiation when normal human fibroblasts were treated with a low dose of UVC (1 J/m(2)). Ataxia telangiectasia (AT), Nijmegen breakage syndrome (NBS), and AT-like disorder fibroblasts, which lack an S checkpoint response when exposed to ionizing radiation, responded normally when exposed to UVC and inhibited replicon initiation. Pretreatment of normal and AT fibroblasts with caffeine or UCN-01, inhibitors of ATR (AT mutated and Rad3 related) and Chk1, respectively, abolished the S checkpoint response to UVC. Moreover, overexpression of kinase-inactive ATR in U2OS cells severely attenuated UVC-induced Chk1 phosphorylation and reversed the UVC-induced inhibition of replicon initiation, as did overexpression of kinase-inactive Chk1. Taken together, these data suggest that the UVC-induced S checkpoint response of inhibition of replicon initiation is mediated by ATR signaling through Chk-1 and is independent of ATM, Nbs1, and Mre11.
Figures




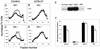
References
-
- Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. - PubMed
-
- Adler, V., S. Y. Fuchs, J. Kim, A. Kraft, M. P. King, J. Pelling, and Z. Ronai. 1995. jun-NH2-terminal kinase activation mediated by UV-induced DNA lesions in melanoma and fibroblast cells. Cell Growth Differ. 6:1437-1446. - PubMed
-
- Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. - PubMed
-
- Blasina, A., B. D. Price, G. A. Turenne, and C. H. McGowan. 1999. Caffeine inhibits the checkpoint kinase ATM. Curr. Biol. 9:1135-1138. - PubMed
-
- Boyer, J. C., W. K. Kaufmann, B. P. Brylawski, and M. Cordeiro-Stone. 1990. Defective postreplication repair in xeroderma pigmentosum variant fibroblasts. Cancer Res. 50:2593-2598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
