A highly Ca2+-sensitive pool of vesicles is regulated by protein kinase C in adrenal chromaffin cells
- PMID: 12446844
- PMCID: PMC139269
- DOI: 10.1073/pnas.242624699
A highly Ca2+-sensitive pool of vesicles is regulated by protein kinase C in adrenal chromaffin cells
Abstract
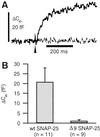
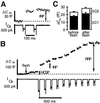
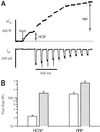
We have used flash photolysis of caged Ca2+ and membrane capacitance measurements to probe exocytosis in chromaffin cells at low concentrations of intracellular Ca2+ ([Ca2+]i) (<10 microM). We observed a small pool of granules that is more sensitive to [Ca2+]i than the previously described "readily releasable pool." Upon activation of PKC, this "highly Ca2+-sensitive pool" is enhanced in size to a greater extent than the readily releasable pool but is eliminated upon expression of a C-terminal deletion mutant (Delta9) of synaptosome-associated protein of 25 kDa (SNAP-25). Thus, in chromaffin cells, PKC enhances exocytosis both by increasing the number of readily releasable vesicles and by shifting vesicles to a highly Ca2+-sensitive state, enabling exocytosis at sites relatively distant from Ca2+ channels.
Figures

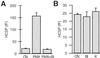
Comment in
-
Specificity emerges in the dissection of diacylglycerol- and protein kinase C-mediated signalling pathways.Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):16522-3. doi: 10.1073/pnas.022708199. Epub 2002 Dec 16. Proc Natl Acad Sci U S A. 2002. PMID: 12486246 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

