Tracing myelin protein zero (P0) in vivo by construction of P0-GFP fusion proteins
- PMID: 12450416
- PMCID: PMC139994
- DOI: 10.1186/1471-2121-3-29
Tracing myelin protein zero (P0) in vivo by construction of P0-GFP fusion proteins
Abstract
Background: Mutations in P0, the major protein of the myelin sheath in peripheral nerves, cause the inherited peripheral neuropathies Charcot-Marie-Tooth disease type 1B (CMT1B), Dejerine-Sottas syndrome (DSS) and congenital hypomyelination (CH). We reported earlier a de novo insertional mutation c.662_663GC (Ala221fs) in a DSS patient. The c.662_663GC insertion results in a frame shift mutation Ala221fs altering the C-terminal amino acid sequence. The adhesion-relevant intracellular RSTK domain is replaced by a sequence similar to Na+/K+ ATPase. To further clarify the molecular disease mechanisms in this sporadic patient we constructed wild type P0 and the c.662_663GC mutant expression cassettes by site-specific mutagenesis and transfected the constructs into insect cells (S2, High5). To trace the effects in live cells, green fluorescent protein (GFP) has been added to the carboxyterminus of the wild type and mutated P0 protein.
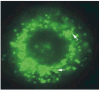
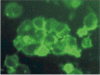
Results: In contrast to the membrane-localized wild type P0-GFP the Ala221fs P0-GFP protein was detectable almost only in the cytoplasm of the cells, and a complete loss of adhesion function was observed.
Conclusions: The present study provides evidence that GFP is a versatile tool to trace in vivo effects of P0 and its mutations. Not only a loss of adhesion function as a result of the loss of the RSTK domain, but also altered intracellular trafficking indicated by a loss of membrane insertion are possible consequences of the Ala221fs mutation.
Figures







Similar articles
-
Clinical phenotypes of different MPZ (P0) mutations may include Charcot-Marie-Tooth type 1B, Dejerine-Sottas, and congenital hypomyelination.Neuron. 1996 Sep;17(3):451-60. doi: 10.1016/s0896-6273(00)80177-4. Neuron. 1996. PMID: 8816708
-
Rapid functional analysis in Xenopus oocytes of Po protein adhesive interactions.Neurochem Res. 2001 Jun;26(6):703-12. doi: 10.1023/a:1010999622760. Neurochem Res. 2001. PMID: 11519730
-
An adhesion test system based on Schneider cells to determine genotype-phenotype correlations for mutated P0 proteins.Genet Anal. 1998 Oct;14(4):117-9. doi: 10.1016/s1050-3862(98)00004-7. Genet Anal. 1998. PMID: 9834852
-
Phenotypic clustering in MPZ mutations.Brain. 2004 Feb;127(Pt 2):371-84. doi: 10.1093/brain/awh048. Epub 2004 Jan 7. Brain. 2004. PMID: 14711881 Review.
-
P0-deficient knockout mice as tools to understand pathomechanisms in Charcot-Marie-Tooth 1B and P0-related Déjérine-Sottas syndrome.Ann N Y Acad Sci. 1999 Sep 14;883:273-80. Ann N Y Acad Sci. 1999. PMID: 10586252 Review.
Cited by
-
Cellular characterization of MPZ mutations presenting with diverse clinical phenotypes.J Neurol. 2010 Oct;257(10):1661-8. doi: 10.1007/s00415-010-5590-8. Epub 2010 May 12. J Neurol. 2010. PMID: 20461396
-
Proteomics of bovine myelin sheath: characterization of a truncated form of P0 by MALDI-TOF/TOF mass spectrometry.J Am Soc Mass Spectrom. 2006 Feb;17(2):117-23. doi: 10.1016/j.jasms.2005.09.011. Epub 2006 Jan 10. J Am Soc Mass Spectrom. 2006. PMID: 16406810
-
Charcot-Marie-Tooth disease: a novel Tyr145Ser mutation in the myelin protein zero (MPZ, P0) gene causes different phenotypes in homozygous and heterozygous carriers within one family.Neurogenetics. 2003 Aug;4(4):191-7. doi: 10.1007/s10048-003-0153-0. Epub 2003 Jul 5. Neurogenetics. 2003. PMID: 12845552
References
-
- Warner LE, Roa BB, Lupski JR. Settling the myelin protein zero question in CMT1B. Nat Genet. 1995;11:119–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

