Effect of different NADH oxidase levels on glucose metabolism by Lactococcus lactis: kinetics of intracellular metabolite pools determined by in vivo nuclear magnetic resonance
- PMID: 12450858
- PMCID: PMC134407
- DOI: 10.1128/AEM.68.12.6332-6342.2002
Effect of different NADH oxidase levels on glucose metabolism by Lactococcus lactis: kinetics of intracellular metabolite pools determined by in vivo nuclear magnetic resonance
Abstract
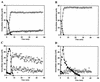
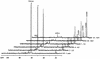
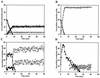
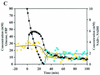
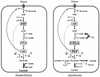
Three isogenic strains of Lactococcus lactis with different levels of H(2)O-forming NADH oxidase activity were used to study the effect of oxygen on glucose metabolism: the parent strain L. lactis MG1363, a NOX(-) strain harboring a deletion of the gene coding for H(2)O-forming NADH oxidase, and a NOX(+) strain with the NADH oxidase activity enhanced by about 100-fold. A comprehensive description of the metabolic events was obtained by using (13)C nuclear magnetic resonance in vivo. The most noticeable results of this study are as follows: (i) under aerobic conditions the level of fructose 1,6-bisphosphate [Fru(1,6)P(2)] was lower than the level under anaerobic conditions, and the rate of Fru(1,6)P(2) depletion was very high; (ii) the levels of 3-phosphoglycerate and phosphoenolpyruvate were considerably enhanced under aerobic conditions and significantly lower in the NOX(-) strain; and (iii) the glycolytic flux decreased in the presence of saturating levels of oxygen, but it was not altered in response to changes in the NADH oxidase activity. In particular, the observation that the glycolytic flux was not enhanced in the NOX(+) strain indicated that glycolytic flux was not primarily determined by the level of NADH in the cell. The patterns of end products were identical for the NOX(-) and parent strains; in the NOX(+) strain the carbon flux was diverted to the production of alpha-acetolactate-derived compounds, and at a low pH this strain produced diacetyl at concentrations up to 1.6 mM. The data were integrated with the goal of identifying the main regulatory aspects of glucose metabolism in the presence of oxygen.
Figures


References
-
- Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44:276-287. - PubMed
-
- Bergersen, F. J., and G. L. Turner. 1979. Systems utilizing oxygenated leghemoglobin and myoglobin as sources of free dissolved O2 at low concentrations for experiments with bacteria. Anal. Biochem. 96:165-174. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

