UTP-dependent inhibition of Na+ absorption requires activation of PKC in endometrial epithelial cells
- PMID: 12451057
- PMCID: PMC2229560
- DOI: 10.1085/jgp.20028608
UTP-dependent inhibition of Na+ absorption requires activation of PKC in endometrial epithelial cells
Abstract
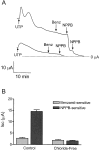
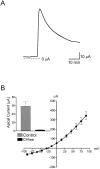
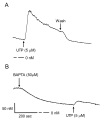
The objective of this study was to investigate the mechanism of uridine 5'-triphosphate (UTP)-dependent inhibition of Na(+) absorption in porcine endometrial epithelial cells. Acute stimulation with UTP (5 microM) produced inhibition of sodium absorption and stimulation of chloride secretion. Experiments using basolateral membrane-permeabilized cell monolayers demonstrated a reduction in benzamil-sensitive Na(+) conductance in the apical membrane after UTP stimulation. The UTP-dependent inhibition of sodium transport could be mimicked by PMA (1 microM). Several PKC inhibitors, including GF109203X and Gö6983 (both nonselective PKC inhibitors) and rottlerin (a PKCdelta selective inhibitor), were shown to prevent the UTP-dependent decrease in benzamil-sensitive current. The PKCalpha-selective inhibitors, Gö6976 and PKC inhibitor 20-28, produced a partial inhibition of the UTP effect on benzamil-sensitive Isc. Inhibition of the benzamil-sensitive Isc by UTP was observed in the presence of BAPTA-AM (50 microM), confirming that activation of PKCs, and not increases in [Ca(2+)](i), were directly responsible for the inhibition of apical Na(+) channels and transepithelial Na(+) absorption.
Figures







Similar articles
-
Insulin stimulates transepithelial sodium transport by activation of a protein phosphatase that increases Na-K ATPase activity in endometrial epithelial cells.J Gen Physiol. 1999 Oct;114(4):561-74. doi: 10.1085/jgp.114.4.561. J Gen Physiol. 1999. PMID: 10498674 Free PMC article.
-
Activation of chloride secretion by isoflavone genistein in endometrial epithelial cells.Cell Physiol Biochem. 2013;32(5):1473-86. doi: 10.1159/000356584. Cell Physiol Biochem. 2013. PMID: 24296547
-
UTP-preferring P2 receptor mediates inhibition of sodium transport in porcine thyroid epithelial cells.Br J Pharmacol. 1999 Aug;127(8):1787-92. doi: 10.1038/sj.bjp.0702733. Br J Pharmacol. 1999. PMID: 10482908 Free PMC article.
-
UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia.Am J Physiol. 1999 Apr;276(4):C827-37. doi: 10.1152/ajpcell.1999.276.4.C827. Am J Physiol. 1999. PMID: 10199813
-
P2Y receptor regulation of K2P channels that facilitate K+ secretion by human mammary epithelial cells.Am J Physiol Cell Physiol. 2018 May 1;314(5):C627-C639. doi: 10.1152/ajpcell.00342.2016. Epub 2018 Jan 24. Am J Physiol Cell Physiol. 2018. PMID: 29365273 Free PMC article.
Cited by
-
Expression of the ZEB1 (deltaEF1) transcription factor in human: additional insights.Mol Cell Biochem. 2008 Nov;318(1-2):89-99. doi: 10.1007/s11010-008-9860-z. Epub 2008 Jul 12. Mol Cell Biochem. 2008. PMID: 18622689
-
Ion transport regulation by P2Y receptors, protein kinase C and phosphatidylinositol 3-kinase within the semicircular canal duct epithelium.BMC Res Notes. 2010 Apr 14;3:100. doi: 10.1186/1756-0500-3-100. BMC Res Notes. 2010. PMID: 20398257 Free PMC article.
-
Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species.FASEB J. 2009 Nov;23(11):3829-42. doi: 10.1096/fj.09-135590. Epub 2009 Jul 13. FASEB J. 2009. PMID: 19596899 Free PMC article.
-
Mechanism of luminal ATP activated chloride secretion in a polarized epithelium.J Physiol Sci. 2019 Jan;69(1):85-95. doi: 10.1007/s12576-018-0623-7. Epub 2018 Jun 14. J Physiol Sci. 2019. PMID: 29949063 Free PMC article.
-
SARS-CoV proteins decrease levels and activity of human ENaC via activation of distinct PKC isoforms.Am J Physiol Lung Cell Mol Physiol. 2009 Mar;296(3):L372-83. doi: 10.1152/ajplung.90437.2008. Epub 2008 Dec 26. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19112100 Free PMC article.
References
-
- Chan, H.C., C.Q. Liu, S.K. Fong, S.H. Law, L.J. Wu, E. So, Y.W. Chung, W.H. Ko, and P.Y. Wong. 1997. Regulation of Cl− secretion by extracellular ATP in cultured mouse endometrial epithelium. J. Membr. Biol. 156:45–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

