Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation but normal critical period for monocular deprivation
- PMID: 12451106
- PMCID: PMC6758729
- DOI: 10.1523/JNEUROSCI.22-23-10072.2002
Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation but normal critical period for monocular deprivation
Abstract
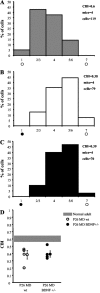
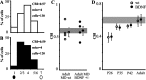
Genetic deletion of a single allele of the BDNF gene affects hippocampal LTP and causes several behavioral phenotypes, including deficits in spatial learning. In the developing visual cortex, overexpression of BDNF accelerates the time course of the critical period for monocular deprivation (MD), and exogenous administration of BDNF alters the outcome of MD. We asked whether reduced levels of BDNF could affect visual cortex plasticity by studying long-term potentiation (LTP) induction and the effects of MD in heterozygous BDNF knock-out mice. We found that theta burst stimulation that induced LTP in the layer IV-III pathway of wild-type (wt) mice caused only a transient potentiation in BDNF+/- mice, and that this potentiation vanished in 25 min. In contrast, LTP elicited by stimulation of the white matter (WM), a form of LTP that can be induced only during the critical period, occurred normally in wt and BDNF+/- mice. The effects of MD during the critical period were similar in wt and BDNF+/- mice, indicating that layer IV-evoked, layer III LTP is not required for ocular dominance plasticity. We then asked whether reduction of cortical BDNF levels could prolong the critical period for MD and for the WM-evoked, layer III LTP induction. We found that in adult BDNF+/- mice, WM-evoked, layer III LTP was not inducible, and that the critical period for MD terminated normally. We conclude that deletion of one copy of the BDNF gene selectively impairs LTP of the layer IV-III pathway but does not alter ocular dominance plasticity.
Figures


References
-
- Akaneya Y, Tsumoto T, Hatanaka H. Brain-derived neurotrophic factor blocks long-term depression in rat visual cortex. J Neurophysiol. 1996;76:4198–4201. - PubMed
-
- Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. - PubMed
-
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. - PubMed
-
- Galuske RA, Kim DS, Castren E, Thoenen H, Singer W. Brain-derived neurotrophic factor reversed experience-dependent synaptic modifications in kitten visual cortex. Eur J Neurosci. 1996;8:1554–1559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
