Potassium currents during the action potential of hippocampal CA3 neurons
- PMID: 12451111
- PMCID: PMC6758734
- DOI: 10.1523/JNEUROSCI.22-23-10106.2002
Potassium currents during the action potential of hippocampal CA3 neurons
Abstract
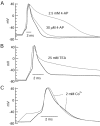
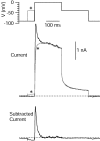
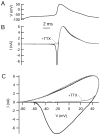
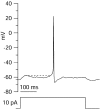
Central neurons have multiple types of voltage-dependent potassium channels, whose activation during action potentials shapes spike width and whose activation and inactivation at subthreshold voltages modulate firing frequency. We characterized the voltage-dependent potassium currents flowing during the action potentials of hippocampal CA3 pyramidal neurons and examined the susceptibility of the underlying channel types to inactivation at subthreshold voltages. Using acutely dissociated neurons that permitted rapid voltage clamp, action potentials recorded previously were used as the command voltage waveform, and individual components of potassium current were identified by pharmacological sensitivity. The overall voltage-dependent potassium current in the neurons could be split into three major components based on pharmacology and kinetics during step voltage pulses: I(D) (fast activating, slowly inactivating, and sensitive to 4-aminopyridine at 30 microm), I(A) (fast activating, fast inactivating, and sensitive to 4-aminopyridine at 3 mm), and I(K) (slowly activating, noninactivating, and sensitive to external TEA at 3-25 mm). The potassium current during the action potential was composed of approximately equal contributions of I(D) and I(A), with a negligible contribution of I(K). I(D) and I(A) had nearly identical trajectories of activation and deactivation during the action potential. Both I(A) and I(D) showed steady-state inactivation at subthreshold voltages, but maximal inactivation at such voltages was incomplete for both currents. Because of the major contribution of both I(D) and I(A) to spike repolarization, it is likely that modulation or partial inactivation at subthreshold voltages of either current can influence spike timing with minimal effect on spike width.
Figures







Similar articles
-
Different mechanisms underlying the repolarization of narrow and wide action potentials in pyramidal cells and interneurons of cat motor cortex.Neuroscience. 1996 Jul;73(1):57-68. doi: 10.1016/0306-4522(96)00010-3. Neuroscience. 1996. PMID: 8783229
-
Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons.J Neurophysiol. 2000 Jan;83(1):70-80. doi: 10.1152/jn.2000.83.1.70. J Neurophysiol. 2000. PMID: 10634854
-
Somatic voltage-gated potassium currents of rat hippocampal pyramidal cells in organotypic slice cultures.J Physiol. 1996 Sep 1;495 ( Pt 2)(Pt 2):367-81. doi: 10.1113/jphysiol.1996.sp021600. J Physiol. 1996. PMID: 8887750 Free PMC article.
-
Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells.J Physiol. 1987 Apr;385:733-59. doi: 10.1113/jphysiol.1987.sp016517. J Physiol. 1987. PMID: 2443676 Free PMC article. Review.
-
The roles of I(D), I(A) and I(K) in the electrophysiological functions of small diameter rat trigeminal ganglion neurons.Curr Mol Pharmacol. 2010 Jan;3(1):30-6. doi: 10.2174/1874467211003010030. Curr Mol Pharmacol. 2010. PMID: 20030627 Review.
Cited by
-
Spike-time precision and network synchrony are controlled by the homeostatic regulation of the D-type potassium current.J Neurosci. 2010 Sep 22;30(38):12885-95. doi: 10.1523/JNEUROSCI.0740-10.2010. J Neurosci. 2010. PMID: 20861392 Free PMC article.
-
Properties of single voltage-dependent K+ channels in dendrites of CA1 pyramidal neurones of rat hippocampus.J Physiol. 2004 Aug 15;559(Pt 1):187-203. doi: 10.1113/jphysiol.2004.068114. Epub 2004 Jun 24. J Physiol. 2004. PMID: 15218076 Free PMC article.
-
Different species, different gap junctions?J Gen Physiol. 2023 Sep 4;155(9):e202313430. doi: 10.1085/jgp.202313430. Epub 2023 Aug 1. J Gen Physiol. 2023. PMID: 37526640 Free PMC article.
-
Aging-Related Hyperexcitability in CA3 Pyramidal Neurons Is Mediated by Enhanced A-Type K+ Channel Function and Expression.J Neurosci. 2015 Sep 23;35(38):13206-18. doi: 10.1523/JNEUROSCI.0193-15.2015. J Neurosci. 2015. PMID: 26400949 Free PMC article.
-
Involvement of GABAergic Interneuron Subtypes in 4-Aminopyridine-Induced Seizure-Like Events in Mouse Entorhinal Cortex in Vitro.J Neurosci. 2023 Mar 15;43(11):1987-2001. doi: 10.1523/JNEUROSCI.1190-22.2023. Epub 2023 Feb 21. J Neurosci. 2023. PMID: 36810229 Free PMC article.
References
-
- Bergles DA. PhD thesis. Stanford University; 1995. The actions of norepinephrine on hippocampal interneurons.
-
- Brown DA, Gahwiler BH, Griffith WH, Halliwell JV. Membrane currents in hippocampal neurons. Prog Brain Res. 1990;83:141–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
