Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase
- PMID: 12451112
- PMCID: PMC6758733
- DOI: 10.1523/JNEUROSCI.22-23-10116.2002
Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase
Abstract
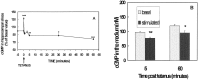
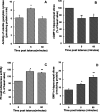
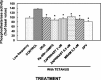
Previous studies indicate that cGMP is involved in long-term potentiation (LTP). However, the effects of application of tetanus to induce LTP on cGMP content and the mechanisms by which cGMP may modulate LTP have not been reported. The aim of this work was to study the time course of the changes in cGMP content and of the activity of soluble guanylate cyclase (sGC) (the enzyme that synthesizes cGMP) during LTP. Moreover, we also studied how the changes in cGMP affect cGMP-dependent protein kinase (PKG) and cGMP-degrading phosphodiesterase and the possible role of these changes in LTP. Application of tetanus induced a rise in cGMP, reaching a maximum 10 sec after tetanus. cGMP content decreased below basal levels 5 min after tetanus and remained decreased after 60 min. Activity of sGC increased 5 min after tetanus and returned to basal at 60 min. Tetanus increased the activity of cGMP-degrading phosphodiesterase at 5 and 60 min. GMP, the product of degradation, was increased at 5 and 60 min. Activation of phosphodiesterase and a decrease in cGMP were prevented by inhibiting PKG with Rp-8-bromoguanosine-cGMPS (Rp-8-Br-cGMPS). Inhibition of sGC [with ODQ (oxadiazolo quinoxalin-1-one) or NS 2028 (4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one)], of PKG (with Rp-8-Br-cGMPS), or of cGMP-degrading phosphodiesterase [with zaprinast or MBAM (4-[[3',4'-(methylenedioxy)benzyl]amino]-6-methoxyquinazoline) ] impairs LTP. The results indicate that induction of LTP involves transient activation of sGC and an increase in cGMP, followed by activation of cGMP-dependent protein kinase, which, in turn, activates cGMP-degrading phosphodiesterase, resulting in long-lasting reduction of cGMP content.
Figures


Similar articles
-
Sequential activation of soluble guanylate cyclase, protein kinase G and cGMP-degrading phosphodiesterase is necessary for proper induction of long-term potentiation in CA1 of hippocampus. Alterations in hyperammonemia.Neurochem Int. 2004 Nov;45(6):895-901. doi: 10.1016/j.neuint.2004.03.020. Neurochem Int. 2004. PMID: 15312984 Review.
-
Hyperammonemia impairs long-term potentiation in hippocampus by altering the modulation of cGMP-degrading phosphodiesterase by protein kinase G.Neurobiol Dis. 2004 Feb;15(1):1-10. doi: 10.1016/j.nbd.2003.09.008. Neurobiol Dis. 2004. PMID: 14751765
-
Molecular mechanisms of the alterations in NMDA receptor-dependent long-term potentiation in hyperammonemia.Metab Brain Dis. 2005 Dec;20(4):265-74. doi: 10.1007/s11011-005-7905-5. Metab Brain Dis. 2005. PMID: 16382337 Review.
-
Evidence for involvement of the cGMP-protein kinase G signaling system in the induction of long-term depression, but not long-term potentiation, in the dentate gyrus in vitro.J Neurosci. 1998 May 15;18(10):3589-96. doi: 10.1523/JNEUROSCI.18-10-03589.1998. J Neurosci. 1998. PMID: 9570790 Free PMC article.
-
On the role of nitric oxide in hippocampal long-term potentiation.J Neurosci. 2003 Mar 1;23(5):1941-8. doi: 10.1523/JNEUROSCI.23-05-01941.2003. J Neurosci. 2003. PMID: 12629199 Free PMC article.
Cited by
-
Visinin-like protein-1 level is associated with short-term functional outcome of acute ischemic stroke: A prospective cohort study.Medicine (Baltimore). 2020 Feb;99(9):e19252. doi: 10.1097/MD.0000000000019252. Medicine (Baltimore). 2020. PMID: 32118731 Free PMC article.
-
Circadian gating of neuronal functionality: a basis for iterative metaplasticity.Front Syst Neurosci. 2014 Sep 19;8:164. doi: 10.3389/fnsys.2014.00164. eCollection 2014. Front Syst Neurosci. 2014. PMID: 25285070 Free PMC article. Review.
-
Amyloid-β Peptide Is Needed for cGMP-Induced Long-Term Potentiation and Memory.J Neurosci. 2017 Jul 19;37(29):6926-6937. doi: 10.1523/JNEUROSCI.3607-16.2017. Epub 2017 Jun 16. J Neurosci. 2017. PMID: 28626017 Free PMC article.
-
cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action.Pharmacol Rev. 2010 Sep;62(3):525-63. doi: 10.1124/pr.110.002907. Pharmacol Rev. 2010. PMID: 20716671 Free PMC article. Review.
-
Phosphatidylinositol 3-kinase/protein kinase Cdelta activation induces close homolog of adhesion molecule L1 (CHL1) expression in cultured astrocytes.Glia. 2010 Feb;58(3):315-28. doi: 10.1002/glia.20925. Glia. 2010. PMID: 19672967 Free PMC article.
References
-
- Akers RF, Lovinger DM, Colley PA, Linden DJ, Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986;231:587–589. - PubMed
-
- Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′, 5′-cyclic GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. - PubMed
-
- Baginski ES, Foá PP, Zak B. Glucose-6-phosphatase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Verlag Chemie Weinheim; Weinheim, Germany: 1974. pp. 876–880.
-
- Beltman J, Sonnenburg WK, Beavo JA. The role of protein phosphorylation in the regulation of cyclic nucleotide phosphodiesterases. Mol Cell Biochem. 1993;127–128:239–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
