Prolonged activation of Ca2+-activated K+ current contributes to the long-lasting refractory period of Aplysia bag cell neurons
- PMID: 12451114
- PMCID: PMC6758731
- DOI: 10.1523/JNEUROSCI.22-23-10134.2002
Prolonged activation of Ca2+-activated K+ current contributes to the long-lasting refractory period of Aplysia bag cell neurons
Abstract
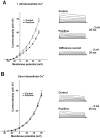
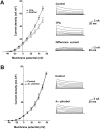
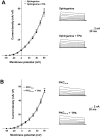
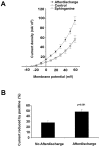
Stimulation of the bag cell neurons of Aplysia activates several biochemical pathways, including protein kinase C (PKC), and alters their excitability for many hours. After an approximately 30 min afterdischarge, these neurons enter an approximately 18 hr inhibited state during which additional stimulation fails to evoke discharges. In vivo, this refractory period limits the frequency of reproductive behaviors associated with egg laying. We have now examined the role of Ca2+-activated K+ (BK) currents in the refractory period. Outward currents gated by both intracellular Ca2+ and depolarization, with pharmacological characteristics of BK currents, were recorded in isolated bag cell neurons. These currents were enhanced by the BK channel activators phloretin and 1,3-dihydro-1-[2-hydroxy-5-(trifluoro-methyl)phenyl]-5-trifluoromethyl-2H-benzimidazol-2-one and inhibited by the BK blocker paxilline. The BK component of K+ current was enhanced by 12-O-tetradecanoyl-phorbol-13-acetate, an activator of PKC, and this effect was blocked by sphinganine and PKC(19-36), inhibitors of PKC in bag cell neurons. To test whether the BK current is altered during the refractory period, intact clusters were stimulated to afterdischarge, and neurons were isolated after the clusters had entered the refractory period. Compared with unstimulated cells, current density was almost doubled in refractory neurons. This increase in current was inhibited by preincubating clusters in sphinganine. Treatment of refractory clusters with paxilline significantly restored the ability of stimulation to evoke afterdischarges. Conversely, application of phloretin to previously unstimulated clusters inhibited the onset of afterdischarges. These results indicate that a prolonged increase in BK channel activity contributes to the prolonged refractory period of the bag cell neurons.
Figures



Similar articles
-
Diacylglycerol-mediated regulation of Aplysia bag cell neuron excitability requires protein kinase C.J Physiol. 2016 Oct 1;594(19):5573-92. doi: 10.1113/JP272152. Epub 2016 Jun 30. J Physiol. 2016. PMID: 27198498 Free PMC article.
-
Activation of a Ca2+-permeable cation channel produces a prolonged attenuation of intracellular Ca2+ release in Aplysia bag cell neurones.J Physiol. 2000 Jan 15;522 Pt 2(Pt 2):271-83. doi: 10.1111/j.1469-7793.2000.t01-2-00271.x. J Physiol. 2000. PMID: 10639103 Free PMC article.
-
Calcium entry causes a prolonged refractory period in peptidergic neurons of Aplysia.J Neurosci. 1983 Nov;3(11):2230-9. doi: 10.1523/JNEUROSCI.03-11-02230.1983. J Neurosci. 1983. PMID: 6631477 Free PMC article.
-
Large-conductance voltage- and Ca2+-activated K+ channel regulation by protein kinase C in guinea pig urinary bladder smooth muscle.Am J Physiol Cell Physiol. 2014 Mar 1;306(5):C460-70. doi: 10.1152/ajpcell.00325.2013. Epub 2013 Dec 18. Am J Physiol Cell Physiol. 2014. PMID: 24352333 Free PMC article.
-
Electric currents applied during the refractory period can modulate cardiac contractility in vitro and in vivo.Heart Fail Rev. 2001 Jan;6(1):27-34. doi: 10.1023/a:1009851107189. Heart Fail Rev. 2001. PMID: 11248765 Review. No abstract available.
Cited by
-
Nicotine inhibits potassium currents in Aplysia bag cell neurons.J Neurophysiol. 2016 Jun 1;115(5):2635-48. doi: 10.1152/jn.00816.2015. Epub 2016 Feb 10. J Neurophysiol. 2016. PMID: 26864763 Free PMC article.
-
Regulation of an Aplysia bag-cell neuron cation channel by closely associated protein kinase A and a protein phosphatase.J Neurosci. 2004 Jul 28;24(30):6833-41. doi: 10.1523/JNEUROSCI.1694-04.2004. J Neurosci. 2004. PMID: 15282289 Free PMC article.
-
PKC enhances the capacity for secretion by rapidly recruiting covert voltage-gated Ca2+ channels to the membrane.J Neurosci. 2015 Feb 11;35(6):2747-65. doi: 10.1523/JNEUROSCI.3581-14.2015. J Neurosci. 2015. PMID: 25673863 Free PMC article.
-
Diacylglycerol-mediated regulation of Aplysia bag cell neuron excitability requires protein kinase C.J Physiol. 2016 Oct 1;594(19):5573-92. doi: 10.1113/JP272152. Epub 2016 Jun 30. J Physiol. 2016. PMID: 27198498 Free PMC article.
-
Association/dissociation of a channel-kinase complex underlies state-dependent modulation.J Neurosci. 2005 Aug 31;25(35):8037-47. doi: 10.1523/JNEUROSCI.1903-05.2005. J Neurosci. 2005. PMID: 16135761 Free PMC article.
References
-
- Aoki T, Baraban SC. Properties of a calcium-activated K+ current on interneurons in the developing rat hippocampus. J Neurophysiol. 2000;83:3453–3461. - PubMed
-
- Atkinson, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
-
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hkCNMB3 and hkCNMB4. J Biol Chem. 2000;275:6453–6461. - PubMed
-
- Chung SK, Reinhart PH, Martin BH, Brautigan DL, Levitan IB. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991;253:560–562. - PubMed
-
- Cibulsky SM, Zhou Y, Daniele LL, Yokoyama CT, Catterall WA, Levitan IB. Association between calcium-activated potassium channels and voltage-gated calcium channels. Soc Neurosci Abstr. 2001;31:139.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
